More Information
Submitted: June 10, 2021 | Approved: June 23, 2021 | Published: June 24, 2021
How to cite this article: Masoomi SR, Azizi M, Aghlmand R, Gheibi M, Kian Z. Simulating the dispersion of poisonous organic chemical compounds in wastewater treatment process through the active sludge method using the TOXChem model. Ann Biomed Sci Eng. 2021; 5: 025-031.
DOI: 10.29328/journal.abse.1001014
ORCiD: orcid.org/0000-0003-1987-5790
Copyright License: © 2021 Masoomi SR, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Keywords: Wastewater treatment plants; poisonous organic chemical compounds; Simulation; TOXChem4.1; Active sludge system
Simulating the dispersion of poisonous organic chemical compounds in wastewater treatment process through the active sludge method using the TOXChem model
Seyyed Roohollah Masoomi1, Mostafa Azizi2, Reza Aghlmand3, Mohammad Gheibi3* and Zahra Kian4
1Department of Environmental Engineering, Civil & Environmental Engineering Faculty, Tarbiat Modares University, Tehran, P.O. Box 14115-397, Iran
2Department of Mine Engineering, Shahid Bahonar University of Kerman, Iran
3Department of Civil Engineering, Ferdowsi University of Mashhad, Iran
4Department of Chemical Engineering, Amirkabir University of Technology, Tehran, Iran
*Address for Correspondence: Mohammad Gheibi, Department of Civil Engineering, Faculty of Engineering, Ferdowsi University of Mashhad, Iran, Email: [email protected]
Naturally, microorganisms decompose the organic material existing in nature, both in the presence or absence of oxygen. The majority of materials such as poisonous chemical compounds, heavy metals, would prevent the treatment process from taking place, lead to the entry of these contaminants into the environment results in the emergence of numerous diseases. In the present study, using the TOXChem4.1 simulation model, attempts were made to simulate a wastewater treatment plant and then assess the dispersions of contaminants including 1,2-Dimethylnaphthalene, 1,3-Dinitropyrene, 1,6-Dimethylnaphthalene, 1,6-Dinitropyrene, and 17a-ethinylestradiol (EE2) in concentrations of a common scenario. The results of computer simulations showed that the EE2 contaminant is of the highest percentage of decomposition among others, due to its wider chemical structure. Consequently, it is clear that such contaminant is of the highest mass in the sludge exiting the treatment plant. In addition, the results of the simulations demonstrated that the highest volumes of gaseous pollutants take place in the modulation and initial sedimentation units.
Wastewater received from municipalities and communities must be treated to satisfy discharge permits and maximum daily load standards, allowing it to be transferred to receiving water sources, soil, or even reused, following global water policy [1]. Nowadays, biological systems can be considered as an operational solution for the treatment of municipal and industrial wastewater. In this method of treatment, the inlet organic load of the wastewater treatment plant is used as food for microorganisms, and the mass of activated microorganisms in this system consumes, decomposes, and transforms organic matter into simplified materials. In other words, microorganisms act as the central brain and the main part of this system [2]. Therefore, the main purpose of all control models and operation of the wastewater treatment plant (both urban and industrial) is to maintain the kinetic and metabolic conditions of microorganisms during the growth and activity process [3]. One of the most common methods of wastewater treatment is the use of aerobic systems, especially the Activated Sludge method, which is used to treat urban and industrial wastewater, with applications ranging from small package plants for single homes to large facilities serving metropolitan areas. Ardern & Lockett (1914) presented the Activated Sludge AS method to the Society for Chemical Industry at the Grand Hotel in Manchester, England on April 3, 1914, and it was the result of sewage treatment research in the United States and the United Kingdom at the end of the 19th and beginning of the 20th centuries [4-6].
One of the main problems in the operation of biological systems, especially in aerobic processes, is the entry of toxic contaminants (inhibitors) that lead to the destruction of the cellular structure of microorganisms and their enzymes. These destructive contaminants include toxic organic chemicals, toxic inorganic chemicals, heavy metals, and algal toxins [2]. Among these, toxic organic chemicals, as well as causing widespread problems in the process of purification and treatment (Upsetting System), by spreading in the effluent, leads to epidemiological problems [7].
In the literature, there are several examples of simulating wastewater treatment plants. Güçlü, et al. (2010) Have designed three distinct ANN models with a back-propagation method to estimate the Ankara central wastewater treatment plant›s effluent suspended solids (SS), chemical oxygen demand (COD), and aeration tank-mixed liquor suspended solids (MLSS) contents [8]. Hendren, et al. (2013) used a model parsimony approach to develop a mass-balance description of engineered nanomaterial (ENM) function based on a small number of input variables to define release quantities to the environment [9]. To estimate 1-day interval T-N concentration of effluent from a wastewater treatment plant in Ulsan, Korea, Guo, et al. (2015) have applied two machine learning models—artificial neural networks (ANNs) and support vector machines (SVMs) [10]. Principal components analysis (PCA) was used by Wang, et al. (2017) to identify important factors for COD and TP prediction. The factors suggested by PCA were used to predict influent COD and TP using the multiple regression approach. In addition, to simulate the performance of wastewater treatment, a full-scale wastewater treatment plant with a moving bed bioreactor (MBBR) and ballasted separation process was designed [11]. In the research of Nordlander, et al. (2017), the activated sludge process was replaced with a microalgae-activated sludge process in a case study in Sweden. Based on mass and energy balances of biological treatment and sludge handling process steps, the impacts on heat and electricity consumption, as well as carbon dioxide emissions, were analyzed in a system model [12]. Baklouti, et al. (2018) have progressed a univariate statistical methodology that uses an improved particle filtering(IPF)-based multiscale optimized exponentially weighted moving average chart (MS-OEWMA) to enhance the monitoring of wastewater treatment plants [13]. In DaNang City, Vietnam, Nguyen, et al. (2020) conducted a measuring study of the HoaCam wastewater treatment plant (WWTP) using the ASM1 model [14]. de Canete, et al. (2021) Have presented a machine learning-based control technique to optimize both the consumption and the number of regulation violations of a biological WWTP [15]. Abbasi, et al. (2021) have modeled three WWTP based on conventional activated sludge, contact stabilization, and step aeration and assessed them technically and economically using the Zargandeh treatment plant data in Tehran with GPS-X software [16]. Ariff (2021) used WWTP simulation software to simulate an industrial wastewater treatment plant at a petrochemical complex in Terengganu, Malaysia [17].
Many models have been used to predict the behavior of emerging pollutants in sewer networks and wastewater treatment plants, and many of them involve empirical mass transfer coefficient derivations (liquid phase (KL) or the gas phase (KG). BASTE, CINCI, WATER9, TOXChem, and Gostelow are among them. WATER9, Gostelow, and TOXChem are three of the most commonly used models [18].
Several researchers have reported some studies that assess the fate and the dispersion of contaminants in the wastewater treatment plant. For example, Francisco Gómez-Rivera, et al. (2012) have used a laboratory-scale Activated Sludge (AS) system fed with primarily-treated municipal wastewater and nano-CeO2 (55.0 mg Ce/L) to explore the fate of nano-CeO2 throughout municipal wastewater treatment [19]. Almeida, et al. (2013) developed a model to characterize ibuprofen and ketoprofen biodegradation by activated sludge from three different WWTP [20]. In a typical secondary activated sludge WWTP, Wang, et al. [21] have investigated the fate of cyclic volatile methyl siloxanes (cVMS) such as octamethylcyclotetrasiloxane (D4), decamethylcyclopentasiloxane (D5), and dodecamethy-lcyclohexasiloxane (D6). Benis, et al. (2016) have studied styrene (STM) and acrylonitrile (ACN) fates and emissions in wastewater pretreatment units in an ABS production plant [22]. Zhao, et al. (2017) used a Monte Carlo simulation to create a fate model that combined secondary and tertiary treatment processes to investigate the fate of six different antibiotics during distinct treatment processes [23]. Fileni, et al. (2018) have studied the dispersion of air pollutants such as Ammonia (NH3) and Hydrogen Sulphide (H2S) emitted by a municipal WWTP for over one year [24]. Using a computer-based mechanistic model, TOXChem V4.1, Zwain, et al. (2019) analyzed the fate of phenol biodegradation in moving bed biofilm reactor sewage treatment plant (MBBR-STP) [18]. Zwain, et al. (2020) have applied TOXChem simulations to predict hydrogen sulfide fate and emissions from extended aeration activated sludge (EAAS) system in the Muharram Aisha-sewage treatment plant (MA-STP) [25]. A high-rate algal pond (HRAP) in North Sweden was explored in terms of API dispersion and fate in Lindberg, et al. (2021) research utilizing municipal untreated wastewater as a medium [26]. The removal efficiencies and fates of selected ECs (three endocrine disruptors (endocrine-disrupting chemicals (EDCs)— triclosan, bisphenol A, and nonylphenol, and four pharmaceuticals (PhACs)— ketoprofen, diclofenac, naproxen, and ibuprofen) in HRAS systems were investigated in the context of the Koumaki, et al. (2021) research [27]. To date, there have been no reliable researches that modeling and evaluating Poisonous Organic Chemical Compounds in WWTP through the AS Method Using the TOXChem Model. This study intends to simulate and determine the emission of 1,2-Dimethylnaphthalene, 1,3-Dinitropyrene, 1,6-Dimethylnaphthalene, 1,6-Dinitropyrene, and 17a-ethiny-lestradiol (EE2) contaminants in a wastewater treatment plant using the TOXChem4.1 simulation model.
he case study wastewater treatment plant
The quality and quantity of wastewater entering the treatment plant and the concentration of its pollutants are summarized in table 1. In the study area (i.e., industrial wastewater treatment plant of Mashhad city in northeastern Iran) the available important pollutants are the same mentioned in table 1. These contaminants have been identified through various tests on gathered wastewater samples from the case study.
| Table 1: Incoming wastewater quality and quantity, as well as pollutant concentrations. | ||||||||
| Flow Rate (m3.d-1) | Suspended Solid (mg.L-1) | VSS/TSS | T(0C) | 1,2-Dimethylnaphthalene (mg.L-1) |
1,3-Dinitropyrene (mg.L-1) |
1,6-Dimethylnaphthalene (mg.L-1) |
EE2 (mg.L-1) |
1,6-Dinitropyrene (mg.L-1) |
| 5000 | 250 | 75% | 25 | 0.007 | 0.2 | 0.1 | 0.3 | 0.6 |
TOXChem model
Toxchem was created in the early 1990s as a replacement for the EPA’s Water8 (Water9) program, which had limitations such as improved mass transfer methods, sorption of contaminants to solids, and a compound database of peer-reviewed physical, chemical, and biological properties. Toxchem is commonly used to determine VOC air emissions from wastewater intake, storage (preliminary, primary, and secondary), and disposal facilities. VOC pollution concentrations are estimated using site-specific drainage characteristics, contaminant properties, and process design and operation statistics. Toxchem is focused on fundamental mass transfer equations and mass balances, such as stripping and volatilization reduction mechanisms, biodegradation, and sorption [18,25,28]. For all substances, not all of these processes would be active. Only sorption (and/or precipitation, which might be indistinguishable from sorption) would be used to remove heavy metals. Biodegradation and volatilization are the main methods of removing VOCs, with sorption playing a minor role. All three processes are capable of removing some hydrophobic organic compounds to a large degree. Separating photolysis and hydrolysis from biodegradation is also difficult. It can thus be used to predict the fate of any synthetic chemical compounds in WWTP’s under either steady-state or dynamic conditions.
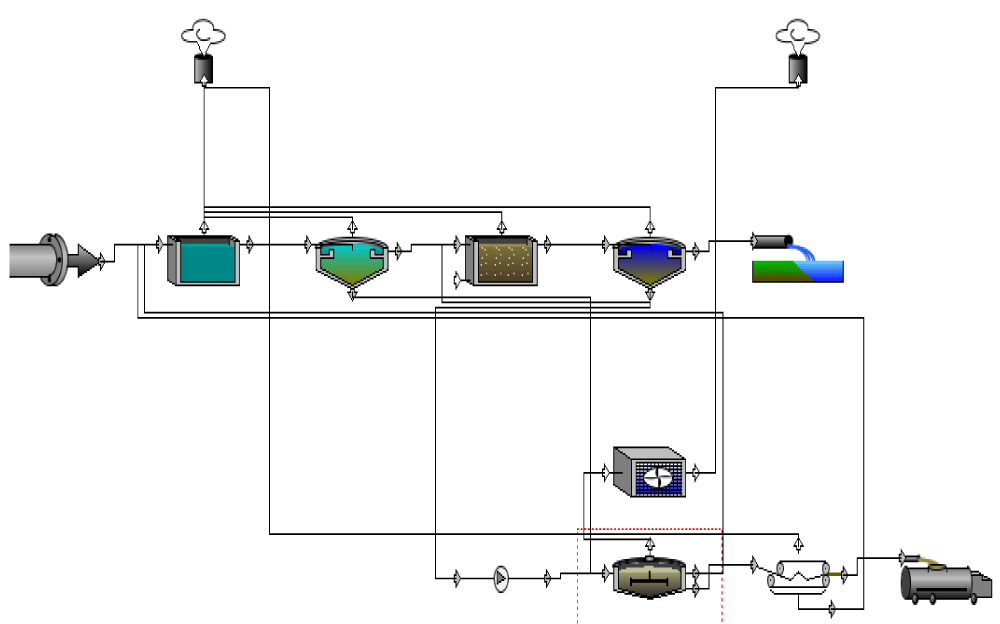
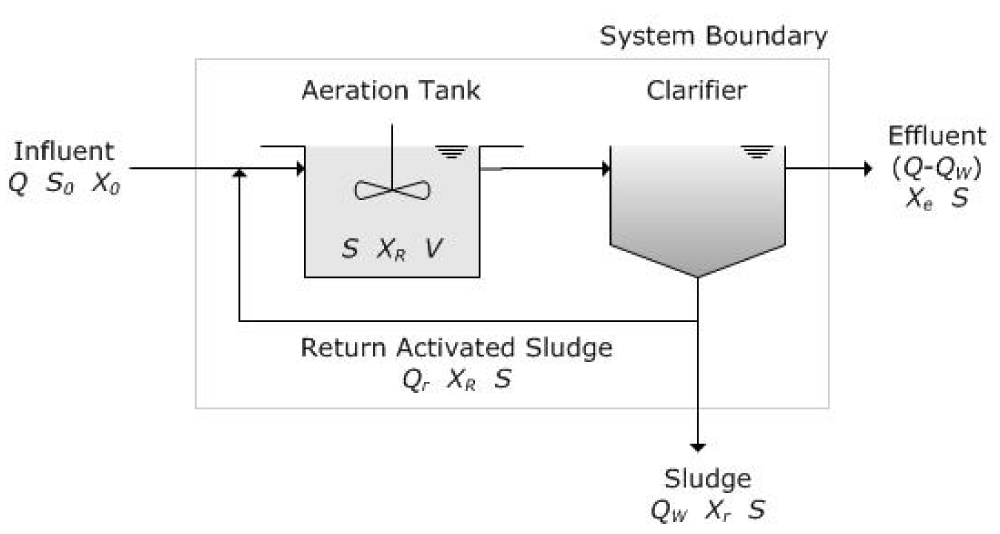
As shown in figure 1, a treatment plant including some different units such as equalization, primary clarifier, Activated Sludge Diffused Aeration (ASDA), secondary clarifier, anaerobic digestion, dewatering by filter press process, and air treatment is modeled. The general pattern of the Activated Sludge Diffused Aeration is shown in figure 2 [29]. According to this figure, generally, S is the substrate, X is the concentration of the microorganism, V is the volume, Q in the flow rate.
Figure 1: The flow diagram of the wastewater treatment plant in the present research.
Figure 2: Biological treatment process by Activated Sludge Diffused Aeration
Contaminants entering the treatment plant
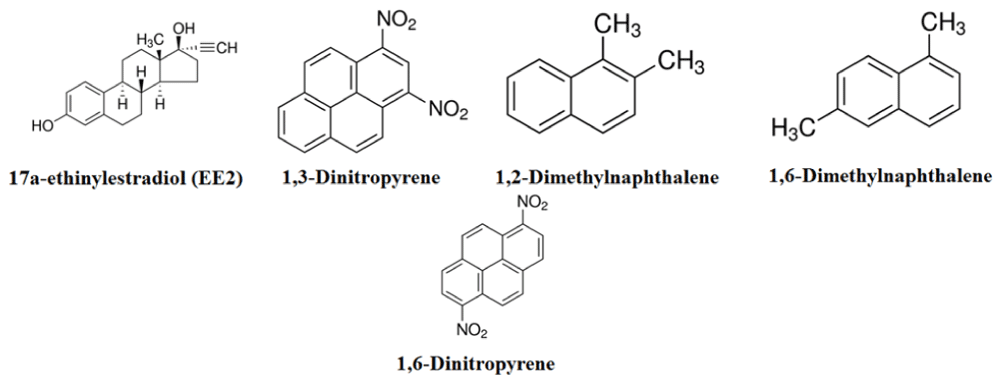
Figure 3 depicts the chemical composition of chemicals entering the investigated wastewater treatment plant [30].
Figure 3: Chemical structure of pollutants entering the wastewater treatment plant.
As previously mentioned, the emission of environmental pollutants (toxic organic chemical compounds) of 1,2-Dimethylnaphthalene, 1,3-Dinitropyrene, 1,6-Dimethylnaphthalene, 1,6-Dinitropyrene, and a-ethinylestradiol17 (EE2) in a wastewater treatment plant was simulated in this research; the technical and qualitative characteristics of the pollutants are described in table 2.
| Table 2: Technical and procedure characteristics of toxins entering the treatment plant under investigation. | |||||
| a-ethinylestradiol17 (EE2) | 1,6-Dinitropyrene | 1,6-Dimethylnaphthalene | 1,3-Dinitropyrene | 1,2-Dimethylnaphthalene | Parameters |
| 296.403 | 292.25 | 156.23 | 292.25 | 156.23 | MW1 |
| 1.21 | 2.02 | 1.0021 | 2.02 | 1.0021 | Density(g.cm-3) |
| 3.24×10E-10 | 3.55×10E-7 | 0.0174 | 3.55×10E-7 | 0.0262 | HL2 |
| 3.67 | 3.84 | 4.26 | 3.84 | 4.31 | LOW/P L3 |
| 4.04×10E-5 | 0.000344 | 0.009283 | 0.000344 | 0.009283 | ABR4 (Kb) |
| 1Molecular Weight (g.mol-1) 2Henrys Law Constant @ 25 C (Lliq.(L.gas)-1) 3Log Octanol/Water part (Log10(Loct.(L.H2O)-1)) 4Aerobic Biodegradable Rate (Kb) @ 20 C in (L.(mg.hr)-1) |
|||||
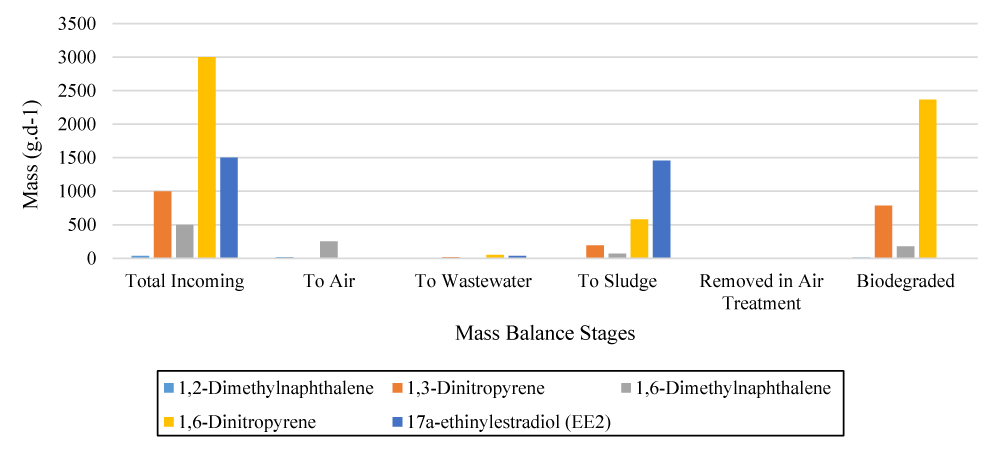
The TOXChem4.1 model was used to simulate the emission of pollutants described in the Materials and Methods section. Figure 4 depicts the results of mass balances for various contaminants.
Dispersion models simulate the fate of gases and airborne particles and help to predict the concentrations of pollutants in the atmosphere. They are important tools in air quality management and planning given that they are cost-effective and time-effective alternatives to field measurements [31]. Figure 4 shows that 1,6-Dimethylnaphthalene pollutants are released into the atmosphere at a higher rate than other pollutants. The results revealed emission and degradation are major processes that occur for 1,6-Dimethylnaphthalene. In Zwain, et al. [25] investigation, the results showed that the key processes happening are: (1) degradation, where the majority of the H2S is oxidized by the aerobic process; and (2) emission, where part of the H2S is released to the atmosphere by H2S stripping and vitalization from open surfaces.
Some parameters can affect the fate and emission of contaminants in wastewater treatment. For example, a sensitivity analysis was conducted in the Zwain, et al. [18] study to better understand the fate of phenol using the most impacting parameters on the treatment process in MBBR systems: influent flow rate, MLSS, and MBBR media fill fraction. Various flow rates (200–1000 m3 per day), MLSS concentrations (500–1000 mg/L), and MBBR medium fill fractions (18% – 88%) were used. Also, sensitivity analysis was used in the Zwain, et al. [25] research to investigate the fate and emission of H2S by applying the major influencing parameters on the treatment process of extended aeration systems, such as aeration flowrate, H2S loading rate (MLSS concentration in the diffused aerated activated sludge reactor), wind speed, wastewater temperature, and wastewater pH level.
Figure 4 also shows EE2 pollutant has the highest mass in total sludge from the primary and secondary clarifier. 1,3-Dinitropyrene contaminant also contains the highest amount of mass degraded by the biological treatment process. The mass values of the emitted pollutants in the atmosphere and in all stages of wastewater treatment are summarized in table 3.
Figure 4: Mass balance of various contaminants at various levels of the wastewater treatment process.
| Table 3: Mass values of different pollutants in all stages of wastewater treatment. | |||||||||
| Air Emissions (g/d) | Air Effluent | Equalization | Primary Clarifier | AS-Diffused | Secondary Clarifier | Air Treatment | Anaerobic Digester | Air Effluent(2) | Belt Filter Press |
| 1,2-Dimethylnaphthalene | 17.61 | 17.14 | 0.45 | 0.01 | 0.0007 | 0.0047 | 0.047 | 0.0047 | 4.008E-05 |
| 1,3-Dinitropyrene | 0.59 | 0.57 | 0.01 | 0.001 | 0.0005 | 4.64E-06 | 4.64E-05 | 4.64E-06 | 3.905E-08 |
| 1,6-Dimethylnaphthalene | 253.49 | 246.86 | 6.48 | 0.134 | 0.0102 | 0.046 | 0.461 | 0.046 | 0.00038 |
| 1,6-Dinitropyrene | 1.77 | 1.72 | 0.04 | 0.003 | 0.0016 | 1.39E-05 | 0.00013 | 1.39E-05 | 1.17E-07 |
| EE2 | 9.16E-10 | 4.13E-10 | 2.05E-10 | 0.000 | 2.78E-10 | 0.000 | 0.000 | 0.000 | 0.000 |
Different contaminants and pollutants emit, and the diversity of chemical pollutants leads to the classification of emissions based on their etiological agent within various types of wastewaters. The rate of emission into the atmosphere is also affected by the design of the sewage channels. As a result of abiotic factors that cause the water to worm and promote more volatilization and release, open wastewaters are more efficiently exhaust emissions than the closed box or underground constructed wastewaters. The following are some of the air contaminants that emerge from wastewater effluents and are simply releasable: 1) Hydrocarbons 2) Volatile compounds 3) Greenhouse gases 4) Airborne microbial contaminants 5) Nitrogen oxides and sulfur oxides 6) Heavy metals [24,32]. Wastewater treatment systems that employ air for separation (air flotation units), for oxygenation (aerobic biological processes), or pollutant removal (air stripping units) will eventually result in the release of VOCs and noxious gases from the wastewater at levels that are potentially harmful to human health [32]. Primary and secondary settlement tanks in wastewater treatment plants are constructed to be quiescent, and in crowded urban treatment plants, these tanks can provide a great surface area. In addition, certain secondary treatment solutions have wide quiescent surfaces, including sequencing batch reactors during the settle and decant phases as well as biological aerated filters awaiting backwash. All of these can be considerable sources of odor emissions in the atmosphere [33]. Regarding table 3, it can be shown that the phases of equalization and Primary Clarifier produce the most gaseous contaminants in all the treatment levels.
Additionally, large quantities of different toxins are released in the form of gas inside the reactors of Activated Sludge and anaerobic digester of sludge. According to Hamoda, (2006) research, the activated sludge aeration tanks produce the highest gaseous emissions, especially when air diffusers are used [34]. Zwain, et al. [18] was observed the phenol emission to air at MBBR-STP in every stage where they were Equalizer (85%), MBBR (12%), and Secondary clarifier (3%), respectively. The results of Zwain, et al. [25] essay showed that emissions from all units were under the human odor threshold (0.0005–1.5 ppm), except for the diffused aerated activated sludge reactor, which had much higher levels.
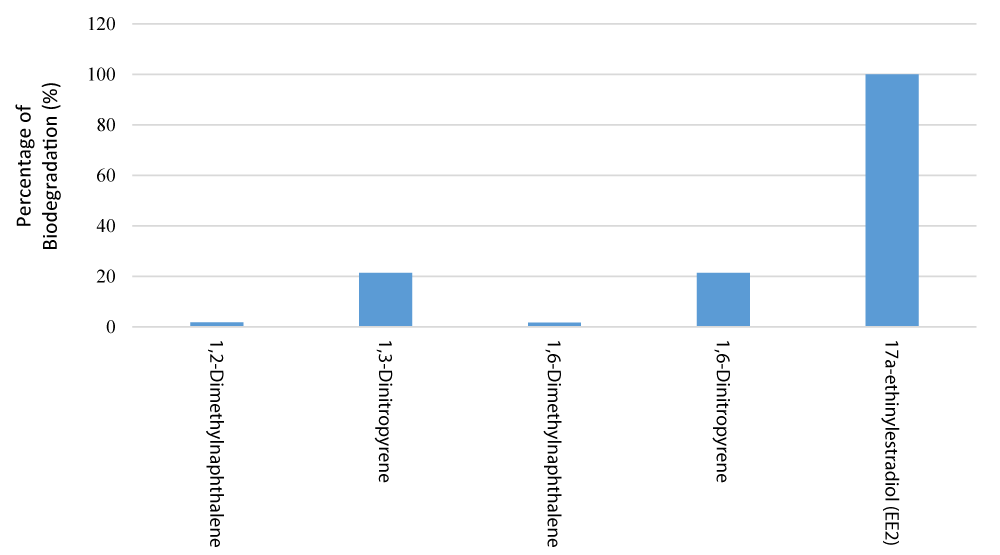
Transfer from open surfaces of tanks, like as clarifiers, is known as surface volatilization. The majority of mass transfer losses are caused by air stripping in aerated turbulent process vessels. Two main assumptions were used in constructing formulations for these transfer processes: Henry’s law and the two-film resistance theory apply. The process of transferring a compound from an area source, such as a primary tank surface and the surface of diffused aerated activated sludge reactor, to the atmosphere is known as volatilization [25,33]. Because the overall mass transfer coefficient plays such a significant role in determining volatilization rates, it must be accurately analyzed. For nonaerated quiescent surfaces, volatilization is usually modeled based on Fick’s law of molecular diffusion and Henry’s law. The mass transfer is regulated by either the liquid phase (KKL) or the gas phase (KG) for a particular Henry’s law coefficient. TOXChem uses the formulas to calculate KKKL and KKG. These formulations take the friction velocity and ScL or ScG into account, but they don’t employ different formulae to account for the fetch-to-depth (F/D) ratio. ScL and ScKKG are the Schmidt number for the liquid phase and the Schmidt number of the gas phase, respectively [33]. Figure 5 summarizes the biodegradability values of the contaminants investigated throughout this review.
Figure 5: Biodegradability rate of organic pollutants entering the treatment plant.
The conventional aerobic and anaerobic treatment systems are unable to degrade all chemicals or convert them to biomass [27]. It has been shown that the biodegradation rate, rKKb, can be represented as a mixed second-order reaction at low substrate concentrations. At a low substrate concentration, C, the Monod equation, and other rate expressions could be approximated by a mixed second-order rate expression, as shown below.
rb = kbXmC (1)
Where Xm = the concentration of mixed liquor volatile suspended solids and kb = the biodegradation rate coefficient. As seen in figure 5, EE2 is more degraded (99.97%) due to its larger complexity and structure than other pollutants. The contaminants 1,3-Dinitropyrene and 1,6-Dinitropyrene, which have a degradation rate of 21.43%, are placed in the next group. Eventually, 1,2-Dimethylnaphthalene and 1,6-Dimethylnaphthalene contaminants were decomposed with 1.82% and 1.69%, respectively. The larger the structure of the organic pollutant leads to microorganisms breaking it down into organic matter easier.
Due to excretion and disposal, the synthetic estrogen 17a-ethinylestradiol (EE2), has been identified in wastewaters and surface waters at ng/L amounts, which can reach up to 50% of the ingested amount. The presence of EE2 in the aquatic environment is problematic since it is classed as a toxic substance to aquatic organisms, capable of causing long-term (chronic) effects such as endocrine disruption and reproductive problems [35,36]. Some applications of EE2 are delay in sexual maturity, decrease in secondary sexual characteristics, and sex determination alteration. Because of its toxicity and durability, EE2 is a contaminant of emerging concern, as it has a substantial impact on living organisms’ metabolism [37].
Hamoda (2006) shows that nonchlorinated compounds such as p-xylene, 4-ethyl toluene, toluene, and 1,3,5-trimethyl benzene were degraded the most and stripped the least.
More than 80% of the mass flow of these chemicals in the influent was biodegraded, while only 20% was stripped. Other VOCs, such as chlorinated compounds, chloroform, dichloromethane, 1,1,1-trichloroethane, trichloroethylene, 1,4-dichlorobenzene, and tetrachloroethylene, degraded less and were stripped more. Stripping for these compounds resulted in losses ranging from 31.1% to 70.8%, with an average of 47.3%. Biodegradation rates varied from 21% to 63%, with a 45% average.
A continuously aerated submerged fixed-bed bioreactor was employed in Forrez, et al. (2009) research for the biological removal of EE2 at µg L-1 levels, with removal efficiencies above 96% [36]. Larcher and Yargeau, [35] studies on heterotrophic bacteria effect on synthetic estrogen biodegradation have performed the Rhodococcus species were the most successful with 38% - 61% EE2 removal after 300 h (R. zopfii, R. erythropolis, R. equi) and no detectable EE2 after only 48 h (R. rhodochrous). EtOH provided an additional carbon source for the bacteria, and it’s probable that EtOH produced greater EE2 degradation via cometabolism in R. rhodochrous. The two mixed bacterial groups investigated, which included 5 (Group 1; no B. subtilis or R. zopfii) and 6 (Group 2; no P. aeruginosa) from the above listed bacterial species, were not able to match these significant EE2 reductions. After 300 hours, the mixed groups of bacteria achieved maximum average EE2 removals of 43 ± 4% (Group 1) and 42 ± 2% (Group 2), respectively. Zwain, et al. [25] also concluded that using the Extended Aeration Activated Sludge (EAAS) system at the lowest aeration flowrate reduces odorant emissions and improves biodegradation treatment.
Due to the development of biological treatment in wastewater treatment plants, the need to control inhibitory factors on the treatment process and also to examine the release of toxic pollutants is of particular importance. At first, this study investigated the emission of 1,2-Dimethylnaphthalene, 1,3-Dinitropyrene, 1,6-Dimethylnaphthalene, 1,6-Dinitropy-rene, and 17a-ethinylestradiol (EE2) contaminants in a wastewater treatment plant using the TOXChem4.1 simulation model. The biological treatment procedure in this treatment plant is carried out using an Activated Sludge Diffused Aeration (ASDA). The results of mass balance research and calculations showed that EE2 and 1,6-Dimethylnaphthalene pollutants have the highest masses in sludge mass and atmosphere, respectively. It was further found that EE2 pollutant is degraded better than other pollutants in this study due to its large chemical structure with a degradation rate of 99.97%.
- Karpinska AM, Bridgeman J. CFD-aided modelling of activated sludge systems–A critical review. Water Res. 2016; 88: 861-879. PubMed: https://pubmed.ncbi.nlm.nih.gov/26615385/
- Inc. Metcalf Eddy. Wastewater engineering: collection, treatment, disposal. McGraw-Hill. 1972.
- Grady Jr, CL, Daigger GT, Love NG, Filipe CD. Biological wastewater treatment. CRC press. 2011.
- Henze M, Harremoes P, la Cour Jansen J, Arvin E. Wastewater treatment: biological and chemical processes: (2002). Springer. 1995.
- Stensel HD, Makinia J. Activated sludge process development. Activated sludge–100 years and counting. Jenkins, D. and Wanner, J.(eds.). London: IWA Publishing. 2014; 33-47.
- Ardern E, Lockett WT. Experiments on the oxidation of sewage without the aid of filters. J Society Chem Industry. 1914; 33: 523-539.
- Vasconcelos VM, Pereira E. Cyanobacteria diversity and toxicity in a wastewater treatment plant (Portugal). Water Res. 2001; 35: 1354-1357.
- Güçlü D, Dursun Ş. Artificial neural network modelling of a large-scale wastewater treatment plant operation. Bioprocess Biosyst Eng. 2010; 33: 1051-1058. PubMed: https://pubmed.ncbi.nlm.nih.gov/20445993/
- Hendren CO, Badireddy AR, Casman E, Wiesner MR. Modeling nanomaterial fate in wastewater treatment: Monte Carlo simulation of silver nanoparticles (nano-Ag). Sci Total Environ. 2013; 449: 418-425. PubMed: https://pubmed.ncbi.nlm.nih.gov/23454703/
- Guo H, Jeong K, Lim J, Jo J, Kim YM, et al. Prediction of effluent concentration in a wastewater treatment plant using machine learning models. J Environ Sci. 2015; 32: 90-101. PubMed: https://pubmed.ncbi.nlm.nih.gov/26040735/
- Wang X, Ratnaweera H, Holm JA, Olsbu V. Statistical monitoring and dynamic simulation of a wastewater treatment plant: A combined approach to achieve model predictive control. J Environ Manage. 2019; 193: 1-7. PubMed: https://pubmed.ncbi.nlm.nih.gov/28187342/
- Nordlander E, Olsson J, Thorin E, Nehrenheim E. Simulation of energy balance and carbon dioxide emission for microalgae introduction in wastewater treatment plants. Algal Res. 2017; 24: 251-260.
- Baklouti I, Mansouri M, Hamida AB, Nounou H, Nounou M. Monitoring of wastewater treatment plants using improved univariate statistical technique. Process Safety and Environmental Protection. 2018; 116: 287-300.
- Nguyen DH, Latifi MA. Simulation and Optimization of Hoa Cam Wastewater Treatment Plant. In International Conference on Sustainable Development of Water and Environment. 2020; 213-223.
- de Canete JF, del Saz-Orozco P, Gómez-de-Gabriel J, Baratti R, Ruano A, et al. Control and soft sensing strategies for a wastewater treatment plant using a neuro-genetic approach. Comp Chem Eng. 2021; 144: 107146.
- Abbasi N, Ahmadi M, Naseri M. Quality and cost analysis of a wastewater treatment plant using GPS-X and CapdetWorks simulation programs. J Environ Manage. 2021; 284: 111993. PubMed: https://pubmed.ncbi.nlm.nih.gov/33540192/
- Ariff IFM. Application of Inhibition Model to Prevent Nitrification Upset in Petrochemical Wastewater Treatment Plant. In Proceedings of the International Conference on Civil, Offshore and Environmental Engineering. Springer, Singapore. 2021; 81-92.
- Zwain HM, Vakili M, Faris AM, Dahlan I. Modeling the fate of phenol in moving bed biofilm reactor sewage treatment plant. In AWAM International Conference on Civil Engineering. Springer, Cham. 2019; 1643-1653.
- Gómez-Rivera F, Field JA, Brown D, Sierra-Alvarez R. Fate of cerium dioxide (CeO2) nanoparticles in municipal wastewater during activated sludge treatment. Bioresour Technol. 2012; 108: 300-304. PubMed: https://pubmed.ncbi.nlm.nih.gov/22265985/
- Almeida B, Oehmen A, Marques R, Brito D, Carvalho G, et al. Modelling the biodegradation of non-steroidal anti-inflammatory drugs (NSAIDs) by activated sludge and a pure culture. Bioresource Technol. 2013; 133: 31-37. PubMed: https://pubmed.ncbi.nlm.nih.gov/23422300/
- Wang DG, Aggarwal M, Tait T, Brimble S, Pacepavicius G, et al. Fate of anthropogenic cyclic volatile methylsiloxanes in a wastewater treatment plant. Water Res. 2015; 72: 209-217.
- Benis KZ, Shakerkhatibi M, Yousefi R, Kahforoushan D, Derafshi S. Emission patterns of acrylonitrile and styrene around an industrial wastewater treatment plant in Iran. Int J Environ Sci Technol. 2016; 13: 2353-2362.
- Zhao L, Ji Y, Yao J, Long S, Li D, et al. Quantifying the fate and risk assessment of different antibiotics during wastewater treatment using a Monte Carlo simulation. J Cleaner Product. 2017; 168: 626-631.
- Fileni L, Matteucci G, Passerini G, Rizza U. Analysis of air pollutant emissions in a wastewater treatment plant using dispersion models. In WIT Transactions on Ecology and Environment, Air Pollution XXVI. 2018; 230: 219-230.
- Zwain HM, Nile BK, Faris AM, Vakili, M, Dahlan I. Modelling of hydrogen sulfide fate and emissions in extended aeration sewage treatment plant using TOXCHEM simulations. Sci Rep. 2020; 10: 22209. PubMed: https://pubmed.ncbi.nlm.nih.gov/33335267/
- Lindberg RH, Namazkar S, Lage S, Östman M, Gojkovic Z, et al. Fate of active pharmaceutical ingredients in a northern high-rate algal pond fed with municipal wastewater. Chemosphere. 2021; 271: 129763. PubMed: https://pubmed.ncbi.nlm.nih.gov/33736225/
- Koumaki E, Noutsopoulos C, Mamais D, Fragkiskatos G, Andreadakis A. Fate of Emerging Contaminants in High-Rate Activated Sludge Systems. Int J Environ Res Public Health. 2021; 18: 400. PubMed: https://pubmed.ncbi.nlm.nih.gov/33419173/
- Khalid KM. Correlation between Air Quality and Wastewater Pollution. In Air Quality. IntechOpen. 2021.
- Goronszy MC. Biological treatment of wastewater. U.S. Patent. 1987; 4: 663,044.
- Grulich G, Hutton DG, Robertaccio FL, Glotzer HL. Treatment of organic chemicals plant wastewater with the Du Pont pact process. Water. 1973.
- Hamoda MF. Air Pollutant Emissions from Waste Treatment and Disposal Facilities. PubMed: https://www.researchgate.net/publication/297467781_Air_Pollutant_Emissions_from_Waste_Treatment_and_Disposal_Facilities
- Santos JM, Kreim V, Guillot JM, Reis Jr, NC, de Sá LM, et al. An experimental determination of the H2S overall mass transfer coefficient from quiescent surfaces at wastewater treatment plants. Atmospheric Environ. 2012; 60: 18-24.
- Brasil Bernardelli JK, Liz MV, Belli TJ, Lobo-Recio MA, Lapolli FR. Removal of estrogens by activated sludge under different conditions using batch experiments. Bra J Chem Eng. 2015; 32: 421-432.
- Hamoda MF. Air pollutants emissions from waste treatment and disposal facilities J Environ Sci Health A Tox Hazard Subst Environ Eng. 2006; 41: 77-85. PubMed: https://pubmed.ncbi.nlm.nih.gov/16401572/
- Larcher S, Yargeau V. Biodegradation of 17α-ethinylestradiol by heterotrophic bacteria. Environ Pollut. 2013; 173: 17-22. PubMed: https://pubmed.ncbi.nlm.nih.gov/23195522/
- Forrez I, Carballa M, Boon N, Verstraete W. Biological removal of 17α‐ethinylestradiol (EE2) in an aerated nitrifying fixed bed reactor during ammonium starvation. J Chem Technol Biotechnol. 2009; 84: 119-125.
- Haq I, Raj A. Endocrine-disrupting pollutants in industrial wastewater and their degradation and detoxification approaches. In Emerging and eco-friendly approaches for waste management. Springer, Singapore. 2019; 121-142.