More Information
Submitted: August 29, 2023 | Approved: September 11, 2023 | Published: September 12, 2023
How to cite this article: Bahmanpour A, Mollazadeh-Bajestani M, Ghaffari M, Moztarzadeh F, Sepahvandi A. Hydrogel-Based Formulations for Drug Delivery to the Posterior Segment of the Eye. Ann Biomed Sci Eng. 2023; 7: 038-050.
DOI: 10.29328/journal.abse.1001024
Copyright License: © 2023 Bahmanpour A, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Keywords: Hydrogel; Drug delivery system; Posterior segment; Implant; Age-related macular degeneration; Diabetic macular edema
Hydrogel-Based Formulations for Drug Delivery to the Posterior Segment of the Eye
AmirHossein Bahmanpour1, Maryam Mollazadeh-Bajestani2, Maryam Ghaffari3, Fathollah Moztarzadeh3 and Azadeh Sepahvandi4*
1Biomaterial Group, Biomedical Engineering Department, University of North Carolina at Charlott, Charlott, NC, USA
2School of Medicine, Mashhad University of Medical Sciences, Mashhad, Iran
3Biomaterial Group, Faculty of Biomedical Engineering (Center of Excellence), Amirkabir University of Technology, Tehran, Iran
4Biomaterials and Tissue Engineering Laboratory, Department of Mechanical Engineering, University of South Carolina, Columbia, SC 29208, USA
*Address for Correspondence: Azadeh Sepahvandi, Ph.D. in Biomedical Engineering, Department of Mechanical Engineering, College of Engineering and Computing, University of South Carolina, A112, 300 Main St, Columbia, SC, 29201, USA, Email: [email protected]
Hydrogel-based formulations hold significant promise for treating ocular diseases that impact the posterior segment of the eye. These formulations exhibit the ability to surmount ocular barriers and offer sustained drug release, rendering them efficacious drug delivery systems. This article addresses the challenges linked to treating disorders affecting the posterior eye segment and underscores the imperative for less invasive drug delivery methodologies. We further delve into diverse contemporary ocular dosage forms, encompassing gels, nanostructures, and implants, with a specific emphasis on hydrogels. Hydrogels offer several merits, including precise targeting, sustained release, enhanced bioavailability, and non-invasiveness. Moreover, they curtail the risk of adverse effects and foster patient adherence. An enthralling advancement is the amalgamation of hybrid drug delivery systems, integrating nanoparticles, liposomes, dendrimers, and stimuli-activated nano-systems, with hydrogels for posterior eye ailment treatment. These hybrid nano-systems exhibit promise in enhancing drug stability, prolonging drug release, and pinpointing specific tissues within the posterior segment. We also provide an overview of ongoing clinical trials and approved hydrogel-based drug delivery systems, like Retisert and Ozurdex. These systems have demonstrated efficacy in managing chronic non-infectious uveitis, Age-related Macular Degeneration (AMD), and diabetic macular edema. Nevertheless, challenges persist, including optimizing bioavailability, maintaining drug stability, and implementing personalized treatment approaches. The incessant evolution of gel-based drug delivery systems stands to substantially enhance patients’ quality of life and establish new benchmarks in treating posterior eye diseases. The future of ophthalmology brims with excitement, as gel-based drug delivery systems hold the potential to revolutionize ocular therapies, providing effective remedies for an array of vision-related afflictions.
The eye, with its intricate anatomy and physiology, assumes a pivotal role in human life. However, its vital importance has necessitated the development of protective barriers, ranging from static membranes to dynamic vascular defenses. While these barriers proficiently shield the eye from external stressors and contaminants, they concurrently impede internal disease treatment. Numerous treatments for irreversible vision impairments necessitate targeted action within the ocular site, encompassing conditions like conjunctivitis, glaucoma, diabetic retinopathy, uveitis, edema, Retinitis Pigmentosa (RP), and Retinal Vein Occlusion (RVO). The prospects of nanotechnology and smart materials have spurred researchers to explore highly effective and minimally invasive carriers for ocular drug delivery [1,2]. In the realm of drug delivery, nanostructured drug delivery systems are posited as strategies capable of surmounting ocular barriers, precisely targeting the posterior segment, and augmenting drug permeation through controlled-release mechanisms [3]. The gamut of drug delivery to the eye encompasses various dosage forms: semisolid forms like gels and ointments, solid forms such as ocular inserts, liquids like solutions and suspensions, and intraocular forms including implants and injections. Of particular note is the in-situ hydrogel, a specific type of ocular gel that can manifest as organogel or hydrogel. Hydrogels proffer diverse advantages, encompassing reduced dose concentrations, diminished dosing frequency, heightened patient comfort, and elevated bioavailability owing to prolonged ocular residence and reduced drainage through the nasolacrimal duct. Additionally, they afford facile and cost-effective manufacturing. Nonetheless, hydrogels do entail certain drawbacks, including transient blurred vision, challenges in self-insertion, and potential eyelid adherence. Moreover, their efficacy in improving bioavailability is circumscribed. The classification of hydrogels is multi-faceted, encompassing stimulus type, mechanism, and chemical reaction [4]. As advances in gel-based drug delivery persist, the quality of life for patients stands to markedly improve, ushering in new benchmarks in eye treatment. The trajectory of treating posterior eye disorders hinges upon the synthesis of compound or hybrid nano-systems, amalgamating nanoparticles, hydrogels, liposomes, dendrimers, stimuli-activated nano-systems, and nanocarriers with implants. Staying abreast of these strides confers benefits upon both ophthalmologists and patients, fostering enhanced treatment options for conditions like macular edema. In this review, we elucidate the mechanisms governing drug traversal and novel drug delivery carriers, underscoring their capacity for targeted drug delivery to the posterior eye segment through minimally invasive techniques. The discussion commences by addressing the challenges of drug delivery to the posterior eye region, followed by an exploration of pragmatic drug delivery systems facilitating efficacious drug administration. Concluding the discourse is an investigation into hydrogels, categorized based on stimuli, accompanied by a survey of recent advancements in this arena.
The data for this study were meticulously gathered through an exhaustive review of existing literature, research papers, and studies focused on hydrogel-based formulations for drug delivery to the posterior segment of the eye in the field of ophthalmology. Multiple reputable sources, including peer-reviewed journals, academic publications, conference proceedings, and respected online references, were consulted to compile pertinent data and gain comprehensive insights into the various applications and implications of hydrogel-based drug delivery in ocular health. The process of study selection involved a thorough examination of titles, abstracts, and full texts to ensure that the chosen studies provided valuable insights into this specialized area. Emphasis was placed on selecting studies that presented diverse examples showcasing the applications of hydrogel-based drug delivery in addressing various aspects of ocular health. This rigorous methodology ensures the credibility and reliability of the data presented in this article.
Challenges in delivering drugs to the posterior segment of the eye
The effective delivery of drugs for the treatment of various eye diseases faces significant challenges due to barriers within the posterior segment. This intricate region, encompassing the retina, choroid, and vitreous humor, creates a complex and regulated structure that hinders the optimal distribution of medications. Overcoming these barriers to access the posterior eye segment necessitates innovative strategies. There are two primary methods for administering drugs to this segment, each with distinct advantages and drawbacks. The first method involves topical and systemic administration. Despite their common usage, these methods prove inadequate due to the physiological barriers inherent to the region, resulting in limited bioavailability. Alternatively, intraocular and periocular (subconjunctival, sub-tenon, posterior juxtascleral) routes have been developed to address these challenges [5]. These techniques entail the injection or implantation of drugs through the ocular surface into the intraocular tissues [6,7]. While effective in symptom control, these approaches can be considerably invasive. A comprehensive understanding of these physiological barriers is imperative for the development of innovative solutions. Numerous barriers contribute to the difficulty of achieving therapeutic drug concentrations in the posterior eye segment:
Choroid and bruch’s membrane: Positioned between the sclera and the retina, the choroid and Bruch’s membrane serve as substantial obstacles to drug diffusion. The choroid imposes greater restrictions on the delivery of small molecules compared to the sclera. Choroidal clearance has a relatively minor impact on drug elimination in contrast to the conjunctival route. For instance, when administering 10 mg of Triamcinolone Acetonide (TA) into the sub-tenon space of rabbits, no drug reached the vitreous. This lack of drug presence persisted even after removing choroidal clearance through cryotherapy. However, upon eliminating conjunctival blood and lymph clearance, TA was detected in the vitreous [8].
Blood-retinal barrier (BRB): The BRB consists of two main components: the outer BRB, formed by tight junctions in the Retinal Pigmented Epithelium (RPE), and the inner BRB, comprised of endothelial cells in retinal capillaries. The outer BRB, an epithelial layer with robust tight junctions, prevents passive paracellular drug diffusion. Hydrophobic molecules possess a greater capacity to cross the RPE than hydrophilic molecules due to their ability to diffuse through intracellular routes [9]. The inner BRB lines the microvasculature that supplies the neural retina, creating a robust structural barrier against molecular diffusion to and from the retina [10].
Vitreous and inner limiting membrane: The vitreous, a hydrophilic gel-like fluid that occupies the posterior segment between the retina and the lens, presents a challenge due to its immobility and limited flow. Comprising primarily water (99%), along with collagen fibrils, Hyaluronic Acid (HA), chondroitin sulfate, heparan sulfate, and non-collagenous proteins, the vitreous gel restricts drug movement through diffusion [11]. Clearance from the posterior segment relies on molecules diffusing through the endothelium of retinal blood vessels within the inner BRB and/or the RPE to reach the choriocapillaris. Smaller, more lipophilic molecules experience enhanced posterior clearance compared to larger, hydrophilic molecules [12].
Physicomechanical characteristics for drug delivery to the posterior segment of the eye
Efficiently designing drug delivery systems that target the posterior segment of the eye presents a series of challenges. An integral consideration in this endeavor involves defining the physicochemical attributes of the drug delivery system, a crucial determinant of optimal functionality. This paper underscores the paramount role of hydrogels in delivering drugs to the posterior segment. Notably, hydrogels have garnered significant attention due to their mechanical properties that harmonize with the tissue in the posterior segment. Given the intricacy and sensitivity of this tissue, the capability of hydrogels to facilitate controlled drug release has captured the interest of both scientists and surgeons as promising carriers for posterior drug delivery [13].
When crafting a hydrogel as a drug carrier, the principal emphasis is placed on its proficiency in effectively encapsulating and dispensing drugs, ensuring efficient bioavailability. Hydrogels exhibit the ability to encapsulate highly concentrated drugs and to sustain their gradual release from the crosslinked matrix. Additionally, achieving low hydrogel viscosity emerges as a pivotal factor in their design. This low viscosity guarantees uniform dispersion of the active compound prior to gelation [14]. Maintaining viscosity stability becomes imperative in maximizing therapeutic efficacy, as prevailing ocular hydrogels may witness diminished bioavailability under physiological conditions due to viscosity alterations stemming from temperature shifts and dilution effects [15].
Another pivotal facet is the rheological characteristics of hydrogels, dictating their rigidity. Investigations indicate that augmenting the cross-linker concentration and microsphere loading can heighten hydrogel stiffness, bolstering their ability to withstand tear flushing and prolonging their retention within the ocular cavity [16]. Pan, et al. [17] have pioneered hydrogel-based eye drops and devised two-component biomimetic hydrogels capable of forming in situ, offering customizable mechanical and osmotic attributes akin to the vitreous humor. This innovation augments drug release efficiency and effectiveness. The study underscores that hydrogel applications in treating ocular disorders, leveraging their favorable rheology and viscoelasticity, can effectively counteract tear flushing.
An additional milestone is achieved by Sruthi Santhanam, et al. [18], who have developed a tunable, two-component biomimetic hydrogel with mechanical and osmotic properties resembling the vitreous. Comprising gellan, collagen, and poly(methacrylamide-co-methacrylate), an analog of hyaluronic acid, this hydrogel showcases optical and physical properties mimicking the vitreous. Notably, shear storage moduli ranging from 3 to 358 Pa at 1 Hz and sol-gel transition temperatures spanning 35.5 °C to 43 °C were achieved. This adept manipulation of mechanical attributes, swelling behavior, and transition temperatures facilitates the creation of biocompatible hydrogels possessing properties akin to the vitreous.
Moreover, meticulous regulation of hydrogel degradation significantly influences drug delivery to the posterior segment. Post-injection, hydrogels assume a gel-like structure, with drug release primarily transpiring through gradual hydrogel degradation. Researchers have achieved macroscopic hydrogel degradation control by adjusting ester chemistry and precursor polymer structural parameters, leading to adjustable degradation durations spanning weeks to years [19].
Practical drug delivery systems for effective drug administration to the posterior segment
Treating the posterior segment of the eye presents a significant challenge due to the intricate path drug molecules must navigate to reach their intended targets. Various methods, including non-invasive approaches, have emerged to help drugs overcome barriers within the posterior eye. Among the promising drug delivery systems, implants, nanosystems, and gene therapy stand out. In this section, our focus will be on the gel-based drug delivery system and its potential for drug administration to the posterior segment. In this section, we briefly discuss recent advancements in common drug delivery systems for comparison with new progress on gel-based drug delivery systems, which is the main focus of this review
Vitreous implants: The contemporary landscape offers a diverse array of ocular implants, classified based on their intended applications. Vitreous, characterized as a hydrated gel-like substance, accommodates controlled drug delivery carriers designed to extend drug release. This approach involves minimally invasive surgery to implant carriers within the vitreous [6]. Notably, sustained drug release is achieved through embedding dexamethasone within a matrix of 50:50 poly(lactic-co-glycolic acid) (PLGA), a biodegradable biopolymer. This matrix, as seen in the Ozurdex® implant (Allergan Inc.), maintains therapeutic dexamethasone concentrations for up to 6 months. This implant, with dimensions of 0.46 mm diameter and 6 mm length, features an initial loading dose for 2 months followed by a 4-month plateau release phase [20]. Photoreactive implants offer an innovative strategy for controlling ocular drug delivery. By irradiating photoactive materials, photochemical and photothermal effects are induced to govern drug release, offering precise control without direct carrier contact [21].
Scleral implants: The potential of scleral implants as a more effective and less invasive route for posterior segment drug delivery has emerged. Microneedles (MNs), applied to ocular tissues for over a decade without adverse effects, are promising tools. Polymeric microneedle patches exhibit effectiveness in delivering small molecules, macromolecules, and nanoparticles to the posterior eye. One approach involves applying a microneedle patch to the corneal surface, enabling needle dissolution and subsequent drug release in the corneal epithelium [22,23]. A self-plugging MN (SPM) has been developed to simultaneously facilitate intraocular drug delivery and seal the scleral puncture [24]. The efficacy of microneedle scleral patches (MSPs) in delivering triamcinolone acetonide to the posterior segment has been demonstrated by Roy, et al. [25]. These patches, both Microneedle Scleral (MSP) and Microneedle Corneal (MCP), were fabricated using rapidly dissolvable polyvinylpyrrolidone through the micro-molding technique.
Nano system carriers: Over the past decade, nanotechnology has been explored for sustained drug delivery. This investigation extends to nanostructures’ potential for posterior segment drug delivery. Diverse nanostructures, including Polymeric Nanoparticles (NPs), liposomes, dendrimers, polymeric micelles, inorganic nanoparticles, and carbon nanotubes, encapsulate drugs within their outer shells. Solid Lipid Nanoparticles (SLNs) and Nanostructured Lipid Carriers (NLCs) have exhibited enhanced ocular bioavailability coupled with controlled sustained drug release. These carriers are biocompatible, biodegradable, and sterilizable. SLNs are colloidal systems with a sphere-shaped structure, often used for hydrophilic and hydrophobic drug delivery. Nanostructured Lipid Carriers (NLCs) address SLN limitations, offering more space for drug storage and preventing drug expulsion [26-30].
Gene therapy: Gene therapy, a promising approach for treating posterior eye diseases, remains in its developmental stages. Two main types of gene therapy exist: ex vivo gene therapy involves genetically modifying cells outside the body before re-introducing them, while in vivo gene therapy directly delivers genes to cells within the body. This strategy addresses inherited diseases caused by gene mutations and acquired diseases resulting from damaged genes. Various vectors, including modified viruses and non-viral vectors, are used for gene delivery [31]. Intravitreal injection serves as the primary route for gene therapy administration, with ongoing trials targeting posterior eye diseases. Notable examples include Voretigene neparvovec-rzyl (Luxturna™), approved for treating Leber Congenital Amaurosis (LCA), and RGX-314, aimed at continuous anti-VEGF treatment [32,33]. SAR422459 (EIAV-ABCA4) is undergoing preclinical trials for Stargardt disease, aiming to enhance vision through gene delivery [34]. Cationic polymers are effective methods of gene delivery. Polyethylenimine (PEI) has demonstrated effective transfection of various human cell lines through the proton-sponge effect. However, the main disadvantage of PEI is its limited biocompatibility. There are many studies that attempt to improve biocompatibility including branching of PEI and conjugation with other polymers. Natural polymers, with better biocompatibility, have also been trialed as gene delivery vectors. Liposomes, which are vesicles of lipid with 1 or more phospholipid bilayers enclosing an aqueous core have also demonstrated the ability to deliver the RPE-65 gene into knock-out mice [35].
Direct injection: Intravitreal injection, the most common route, involves injecting drugs directly into the vitreous humor. Although effective, it can cause inflammation and infection. Subconjunctival injection offers a less invasive alternative, involving injection between the conjunctiva and sclera. This approach has fewer side effects but is less effective. The periocular injection targets tissues surrounding the eye, offering even less invasiveness and fewer side effects [36-38].
Gel-based drug delivery system
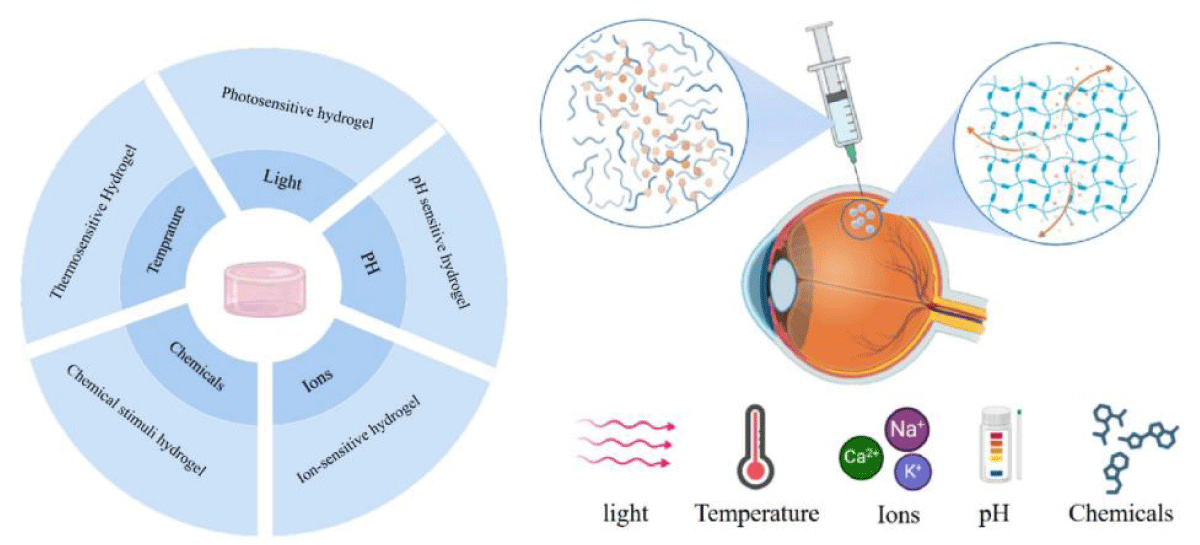
Hydrogels are commonly used as a solution for overcoming drug delivery limitations. They are similar to biological matrices in terms of their high water content, which ranges from 70 to 99% of the gel weight, making them compatible with the vitreous structure in the posterior segment area [39,40]. Hydrogels are semi-solid and not easily injectable into the vitreous. As such, responsive hydrogels that can transition from solution to gel in response to specific stimuli are often used for intravitreal applications [41]. This transition can occur immediately before or upon intravitreal administration, triggered by physiological conditions such as pH, temperature, ionic strength, non-physiological conditions such as light, or an in situ chemical reaction [42]. Figure 1 illustrates different stimuli that might be involved in the gelation process.
Figure 1: Schematic figure represents the sol-gel transition in hydrogel. The sol-gel transition is triggered by specific environmental stimuli, such as changes in temperature, pH, light, ions, or the presence of certain chemicals or biomolecules. When these stimuli are applied, the hydrogel undergoes a phase transition, transforming from a sol into a gel or vice versa. This transition allows the hydrogel to change its physical properties, such as swelling, volume, and mechanical strength, in response to the external triggers. Upon reaching the desired location, the hydrogel can undergo the sol-gel transition, releasing the drug at the target site while minimizing systemic exposure and side effects.
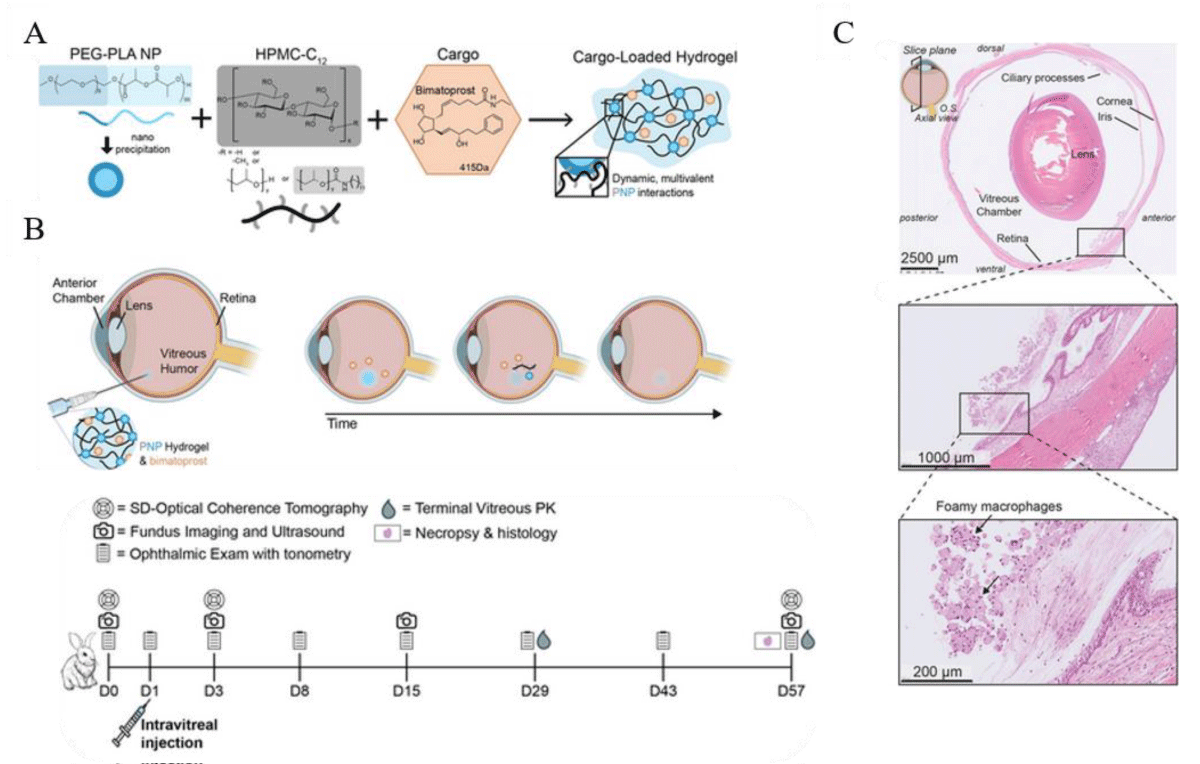
Thermosensitive hydrogels: Thermosensitive hydrogels are a unique type of hydrogel that reacts to temperature changes by altering its physical properties. This makes them an ideal choice for drug delivery to specific areas of the body, such as the eye. Thermosensitive hydrogels are composed of polymers that have a Lower Critical Solution Temperature (LCST). When talking about thermosensitive hydrogels, LCST refers to the temperature that causes the hydrogel to change from a liquid or sol state to a gel state. The LCST is the temperature at which this sol-gel transition occurs. The hydrogel is in a liquid state below the LCST, making it easy to inject into the body. However, once the temperature reaches the LCST, the hydrogel transforms into a gel and forms a depot at the injection site. This change creates a gel matrix with regulated porosity, which allows for the controlled release of drugs contained within the hydrogel [43]. Thermosensitive hydrogels have several advantages for drug delivery to the posterior segment of the eye, including controlled drug release, targeted delivery, and ease of administration. However, challenges such as biocompatibility, drug loading capacity, and stability must be overcome for successful use. In a study conducted by Gado, et al. [44], a chitosan grafted poly(n-isopropylacrylamide) (Cs-g-PNIPAAm) gel was examined for its ability to deliver small molecular weight, multiple tyrosine kinase inhibitor Sunitinib malate in a sustained manner intrasclerally. The hydrogel affected the rheological properties of the hydrogel and the ability to be administered through a syringe. By controlling the temperature of the gelation process and ionic conjugation of sunitinib with the amine group of chitosan, better control over drug release was achieved. The transition temperature was found to be 32 °C ± 0.5 °C and the hydrogel was able to release an approximate concentration of 10 µg/day of sunitinib in-vitro for 28 days. It was observed that the drug was released from the hydrogel through both the diffusion and erosion mechanisms. In animal studies, specifically porcine sclera, up to 40% sustained release of sunitinib was achieved with the hydrogel compared to sunitinib solution (2 mg/ml). Annala, et al. [45] designed an injectable hydrogel delivery system for sustained ocular delivery of dexamethasone. They designed a self-healing hydrogel using a thermosensitive ABA triblock copolymer, covalently linking the drug dexamethasone to the polymer through a copolymerization process. Hydrogel formation occurred through covalent cross-linking at 37 °C, facilitated by the presence of disulfide bonds in the cystamine cross-links, making the system injectable. The hydrogel exhibited a controlled release of dexamethasone over an extended period of 430 days at 37 °C, making it a promising candidate for long-term therapeutic applications. Recently, Meany, et al. [46] conducted a study on vision impairment caused by macular degeneration and glaucoma. They developed a supramolecular Polymer-Nanoparticle (PNP) hydrogel for intravitreal delivery of the glaucoma drug bimatoprost. The PNP hydrogel possesses important properties such as shear-thinning and self-healing, making it easily injectable, and it enables the slow release of the drug in the vitreous humor. In an in vivo study the intravitreally injected PNP hydrogels formed depots that degraded gradually over time, maintaining detectable levels of bimatoprost in the vitreous humor for up to 8 weeks after injection. The researchers emphasized the need to optimize the tolerability of the PNP hydrogel in the eye since it still poses clinical risks like retinal detachment due to the resulting patchy fibroplasia (Figure 2).
Figure 2: Schematic of how bimatoprost-loaded polymer-nanoparticle (PNP) hydrogels are prepared and used to release drugs for a longer period in the vitreous humor (VH) through intravitreal (ITV) injection. A) A mixture of poly(ethylene glycol)-block-poly (lactic acid) nanoparticles (PEG-PLA NPs), HPMC-C12, and bimatoprost is created. Once injected, the hydrogels form a sustained delivery depot that releases bimatoprost gradually over an extended period. B) As time passes, the hydrogel gradually dissolves, releasing the drug and hydrogel components until there is no longer any depot material left. The drug is represented in the schematic by orange hexagons. C)The histopathological examination of rabbit eyes after the administration of PNP hydrogels showed a minimal to mild foreign body response (FBR) in the VH and along the ventral retina. Microscopic observations indicated that the PNP hydrogel caused some impact. An image of the full rabbit eye stained with hematoxylin and eosin (H&E) showed an affected region extending from the ciliary body and along the ventral peripheral retina, with a scale bar of 2500 µm. A closer look at the affected regions revealed the presence of foamy macrophages. Adapted and modified from [46].
Photosensitive hydrogels: Polymers called photoresponsive can turn from liquid to gel when exposed to Near-Infrared (NIR), Visible (VIS), or Ultraviolet (UV) radiation. These types of light can go through the cornea and reach the deeper parts of the eye [47]. As the specific wavelength emits photosensitive hydrogel it crosslinks and leads to gel formation. When the hydrogel is exposed to a certain wavelength of light, it becomes crosslinked and forms a gel. Peng, et al. [48] created a mixture of two types of polymers called AB-DEX and CD-DEX, which respond to UV radiation at 365 nm. When exposed to this light, the AB-DEX polymer changes shape and causes the inclusion complex to break apart, forming a soluble polymer mixture that releases macromolecules. This change can also be used to form a gel in the vitreous of the eye for sustained release. In another study, Bisht, et al. [49] developed a system that combines poly(lactic-co-glycolic) acid nanoparticles (PLGA NPs) with light-responsive injectable implants (ISFIs) for safe delivery of therapeutic peptides to the eye. This composite system can be activated by UV light for 12 minutes and has been shown to be biocompatible in zebrafish embryos. Gelatin methacryloyl, also known as GelMA, has become a popular choice due to its ability to slowly release substances and its low toxicity. In one study, GelMA hydrogels were combined with Triamcinolone Acetonide (TA) and then exposed to light for crosslinking after being injected into the vitreous cavity. The hydrogels quickly formed a gel upon injection, and in vitro testing showed that the TA-hydrogels had a slower and more sustained release of TA compared to TA suspensions [50].
Chemical stimuli hydrogels: In 2001, Nobel laureate K. Barry Sharpless introduced the term “click chemistry” to refer to a group of chemical reactions that can be used to prepare chemically crosslinked hydrogels. These reactions are especially helpful in producing complex molecules with accuracy and control. Click hydrogels have numerous benefits over conventional hydrogel synthesis approaches, including ease of preparation, high efficiency, and the ability to regulate gel properties. Some of the most frequently used click chemistry reactions in making click hydrogels are Azide-Alkyne, Thiol-Ene, and Diels-Alder [51]. Click crosslinking involves a spontaneous reaction and covalent bond formation upon mixing, resulting in the formation of in situ gel. In a study conducted by Yu, et al. [52] Hyaluronic Acid (HA) and Dextran (DEX) were used. They were functionalized with vinyl sulfone and thiol groups, respectively, forming HA-VS and DEX-SH. When these two functionalized polymers were mixed, a “click” reaction occurred between the thiol and alkene functional groups, resulting in the formation of covalent thioether crosslinks between the polymer chains. This hydrogel was found to be well tolerated in rabbit eyes and provided a sustained release of bevacizumab for at least six months in vivo. In another study, Ilochonwu, et al. [53] synthesized a polymer that can undergo a gel transition via Diels-Alder reaction (DA). This DA-crosslinked hydrogel was based on HA and PEG polymers and has potential applications as a long-acting sustained delivery system for bevacizumab and other anti-vascular endothelial growth factor therapeutics. Once the PEG was injected into the posterior segment, the DA reaction was initiated because HA is a polysaccharide that is abundantly present in the vitreous of the eye. The formulation was designed to be injectable into the vitreous cavity using a small needle (29G). After injection, the aqueous polymeric solution formed a crosslinked hydrogel at the administration site, trapping the antibody dissolved in the same solution to obtain an intraocular depot system.
pH-sensitive hydrogels: pH-sensitive hydrogel is designed to respond to changes in pH levels by changing its structure and properties. It remains stable under normal pH conditions but becomes more permeable and prone to releasing substances in acidic or alkaline environments. The composition of pH-sensitive hydrogel involves cross-linked polymer networks that respond to pH variations. The polymer chains contain acidic or basic functional groups, which enable the hydrogel to swell or shrink, change its structure, and affect the release of encapsulated drugs. Some common polymers used in the study of pH-responsive hydrogels are polyacrylate, poly(N-isopropyl acrylamide), and n-vinyl caprolactam [54]. These hydrogels can effectively deliver drugs to the eye over a prolonged period of time. The hydrogels are capable of expanding or contracting in response to changes in pH, which enables them to regulate the release of the drug. Yu, et al. [55] designed and synthesized a stimuli-responsive three-dimensional hydrogel system composed of Carboxymethyl Chitosan (CMC) and poloxamer, using a poly (ethylene oxide)/poly(propylene oxide)/poly(ethylene oxide) (PEO–PPO–PEO) block copolymer. The hydrogel exhibited a reversible sol-gel transition at low concentrations upon changes in temperature and/or pH. The cross-linked hydrogels demonstrated a phase transition in different temperature and pH solutions, with higher swelling observed at 35 °C and pH 7.4 due to larger pores. The release rate of the model drug was highest at 35 °C and pH 7.4, making this novel hydrogel a promising candidate for pH-temperature-responsive ophthalmic drug delivery applications
Ion-sensitive hydrogel: Ion-sensitive systems are designed to respond to changes in the ionic strength of the environment by altering the solution’s viscosity. In situ hydrogels that are ion-sensitive release their drug content when there is a change in the ion concentration of the solution, causing it to solubilize. Essentially, contact between cations (such as Na+, Mg2+, and Ca 2+) in tear solution and electrolytes results in the solution becoming a clear and viscous gel [56]. Gellan gum is a polysaccharide derived from Sphingomonas elodea, suitable for applications like drug delivery, biosensors, and tissue engineering due to its gel-forming properties and biocompatibility. In ion-sensitive hydrogels, gellan gum serves as a key component in the matrix, forming gels in the presence of cations. These hydrogels are sensitive to changes in ion concentration, leading to alterations in swelling and mechanical properties. Moreover, its ease of modification allows for customizing the hydrogel’s responsiveness and tailoring it for specific uses. Xu, et al. [57] conducted a study to formulate an ion-sensitive in situ gel (ISG) of Brimonidine Tartrate (BRT) for patients with open-angle glaucoma and high intraocular pressure. The optimized ISG, containing 2 mg/mL BRT and 0.45% gellan gum, exhibited prolonged retention time and increased bioavailability of the drug in the eye. In vitro release tests showed a quick release and high burst effect of BRT in the ISG. The pharmacokinetic study in rabbit eyes revealed that the BRT-ISG had a 2.7 times higher maximum concentration and 3.4 times greater total exposure to a drug in the eye over a specific time period compared to commercially available BRT eye drops. The ISG formulation provided rapid and sustained release effects, overcoming the drawbacks of the fast loss and short effective time of commercial eye drops. The researchers suggested that this new ISG could potentially become a safer, more effective, and more convenient drug option for delivering drugs into the intraocular part. Alginate is another popular choice for ion-sensitive gels due to its unique properties. It is sensitive to changes in calcium ions, allowing it to form gels in response to specific ions in the environment. Its gel-forming ability with divalent cations, like calcium ions, provides gentle gelation without harsh cross-linking agents. Alginate gels have good mechanical strength and can be engineered for controlled release of ions or substances, making them suitable for sustained drug delivery and tissue engineering applications [58,59]. In Table 1, the most used biomaterials for ocular hydrogels, their nature, crosslinking or gelation process, biodegradability rate, and degradation mechanisms were listed.
| Table 1: Most used biomaterials for ocular hydrogels and their characteristics. | |||||
| Hydrogel Biomaterial | Nature | Crosslinking/Gelation Process | Degradation Duration | Degradation mechanism | Ref |
| Gelatin | Natural polymer derived from collagen | Physical crosslinking induced by temperature change | Moderate (days to weeks) | Enzymatic (collagenase) | [60] |
| Hyaluronic acid | Natural polysaccharides found in eye vitreous | Chemical crosslinking with polymers containing reactive groups | Moderate (days to weeks) | Enzymatic (hyaluronidase) | [61] |
| Chitosan | Derived from partial deacetylation of chitin | Ionic crosslinking using polyanions | Moderate (weeks to months) | Enzymatic (chitinase) | [62] |
| Poly(ethylene glycol) (PEG) | Synthetic polymer | Chemical crosslinking using acrylate end groups | Slow (months to years) | Hydrolytic | [63] |
| Poly(N-isopropylacrylamide) | Synthetic polymer | Physical crosslinking induced by temperature change | Not Biodegradable | N/A | [64] |
| Gellan gum | Microbial extracellular polysaccharide | Ionic crosslinking with cations | Moderate (weeks to months) | Enzymatic (hydrolase) | [65] |
| Alginate | Natural polysaccharides derived from brown algae | Ionic crosslinking with divalent cations like Ca2+ | Moderate (weeks to months) | Enzymatic (alginate lyase) | [66] |
Hybrid hydrogels: By combining different formulations with varying properties, such as degradation rates or responsiveness to external and internal stimuli (e.g., temperature, pH, enzymes), it becomes possible to finely tune the release kinetics of drugs. This allows for sustained drug release over an extended period, reducing the frequency of drug administration and improving patient compliance. One of the limitations of drug delivery with hydrogels is related to the chemical properties of the drugs, particularly drug solubility. Drugs with low solubility may not be easily dispersed or evenly distributed within the hydrogel. As a result, the drug’s release from the hydrogel may be unpredictable and inefficient, leading to suboptimal therapeutic outcomes. To overcome these limitations, researchers may resort to different strategies. Terreni, et al. [67] developed a new potential Ocular Drug Delivery System for the poorly water-soluble drug Cyclosporine-A (CyA) using a combination of in situ gelling systems and self-assembling nanomicellar carriers. They utilized two non-ionic surfactants, VitE-TPGS and RH-40, to produce the nanomicelles. The selected nanomicellar formulation was combined with optimized ion-sensitive polymeric dispersions of gellan gum (GG-LA) to trigger the sol-gel transition. This combined approach resulted in clear aqueous dispersions, easily instilled, which formed a viscous gel when in contact with tear fluid, thereby improving CyA ocular bioavailability. In another study, Chen, et al. [68] developed and optimized an injectable thermosensitive polymeric hydrogel encapsulating Tetramethylpyrazine (TMP) nanoparticles (TMP-NCs-gel) to achieve controlled drug release and high drug-loading. The researchers focused on TMP, a compound known for its multitarget properties, including anti-angiogenic, anti-inflammatory, antioxidant, and anti-fibrotic effects. However, the clinical use of TMP in ocular disorders was hindered due to its lipophilic properties and poor absorption. The hydrogel allowed the sustained release of TMP, both in vitro and in vivo, and demonstrated a porous structure with the ability to transition from liquid to gel phase when the phase transition temperature was reached. Su, et al. [69] developed a novel drug co-delivery system, for enhancing the efficacy of therapy in wet age-related macular degeneration (wAMD). The Bor/RB-M@TRG system consisted of borneol-decorated rhein and baicalein-coloaded microemulsions (Bor/RB-M) as the therapy entity and a temperature-responsive hydrogel matrix as the intravitreal depot. The researchers found that Bor/RB-M exhibited potent in vitro anti-angiogenic effects, possibly due to improved cellular uptake and synergistic actions of rhein and baicalein targeting anti-angiogenic and anti-oxidative stress pathways, respectively. Coumarin-6-labeled Bor/RB-M@TRG (Bor/C6-M@TRG) exhibited deep penetration into the retina and stable accumulation in the Retinal Pigment Epithelium (RPE) layer for at least 14 days. This system holds the potential for addressing the challenges of delivering drugs to the posterior ocular segment and achieving sustained therapeutic effects for wAMD patients. Thermoresponsive hydrogels (e.g. N-isopropylacrylamide, NIPAAM) that collapse in physiological conditions can entrap and sustain the release of a therapeutic protein. However, most Thermoresponsive hydrogels e.g. N-isopropylacrylamide, NIPAAM) are not biodegradable and often require invasive surgery to remove the depot. Adding biodegradable macromolecular to improve the biodegradability of the NIPAAM hydrogel. The crosslinker was designed to enhance the biodegradability of the NIPAAM hydrogel, making it suitable for ocular tissues due to the small size of the eye and the risks associated with non-degradable implants. Acrylated hyaluronic acid is a biodegradable crosslinker that showed a promising strategy for sustained ocular drug delivery, offering new possibilities for delivering proteins and antibodies to the posterior segment of the eye [70]. Cell-based therapy using three-dimensional (3D) polymeric scaffolds that mimic the native Extracellular Matrix (ECM) is one of the choices for developing effective treatments for retinal diseases like AMD. Recently researchers prepared 3D scaffolds using alginate and Bovine Serum Albumin (BSA) with fenofibrate (FNB) using the freeze-drying technique. Incorporating BSA enhanced the scaffold porosity and crosslinking degree, resulting in a robust scaffold suitable for retinal regeneration. The ALG-BSA conjugated scaffolds demonstrated higher FNB loading capacity, slower release in the simulated vitreous humour, and better cell viability and distribution with ARPE-19 cells compared to other scaffold types. The results indicate that ALG-BSA Maillard reaction conjugate scaffolds hold promise for retinal disease treatment and as implantable scaffolds for drug delivery in retinal regenerative applications. The scaffolds demonstrated biodegradability, biocompatibility, and enhanced mechanical stability essential for ocular applications [71]. A dual stimuli-responsive controlled drug delivery system allows for precise and controlled drug release, which can be tailored to the specific needs of the patient or the targeted disease site. The two stimuli used in this context can be either external or internal, and the drug release is typically activated by a combination of these triggers. Targeted drug delivery through different stimuli-responsive mechanisms has been the focus of recent research [72]. Shareef Khan, et al. [73] developed and optimized a novel in situ gelling system for Triamcinolone Acetonide (TAA) delivery, utilizing a dual-responsive approach with reacted tamarind seed xyloglucan (RXG) as the thermoresponsive component and kappa-Carrageenan (κ-CRG) as the ion-sensitive component. The proportions of RXG and κ-CRG in the in situ gels were optimized based on rheological properties, and the resulting formulation exhibited good flow properties at room temperature but transformed into a robust gel in the presence of simulated tear fluid at 35 °C. The in vivo pharmacokinetic studies in rabbits revealed that the optimized dual-responsive in situ gel provided higher and sustained TAA exposure in the vitreous humor compared to a hydroxypropyl-β-cyclodextrin-based aqueous suspension of TAA. This study suggests that this TAA-loaded dual-responsive in situ gel could offer an effective alternative to the intravitreal route for treating posterior uveitis, potentially allowing administration in the precorneal area for improved ocular drug delivery. The latest research on hydride hydrogels is presented in Table 2, outlining their advantages and limitations.
| Table 2: The latest research on hydride hydrogels. | |||||
| Delivery System | Route of Administration | Advantages | Limitations | Cargo | Ref |
| Microsphere-Hydrogel | Intravitreal injection | Single injection | No information on drug distribution and in vivo half-life | Aflibercept | [74] |
| Chitosan grafted hydrogel | Intravitreal injection | Entrapment of small molecules, sustained release 1 month | Challenges in injection | Sunitinib | [75] |
| Supramolecular nanofiber hydrogel | Intravitreal injection | Long-term sustained release | Not specified | Betamethasone phosphate | [76] |
| 3D-printed PHEMA hydrogel | Contact lens | Non-invasive | Limited bioavailability and Short-term efficacy | Avastin | [77] |
| Hydroxyethyl methacrylate and chitosan | Contact lens | Non-invasive | Not specified | Ocular drugs | [78] |
| Hydrogel-coated PLGA-Dotap nanoparticles | Intravitreal injection | Controlled delivery, increased therapeutic efficacy | Limited loading capacity, challenges in injection | Nanoparticle | [79] |
| hydrogel combining chitosan nanoparticles | Intravitreal injection | Avoided the initial burst release | Challenges in injection | dexamethasone/Avastin | [80] |
| Peptide nanofiber hydrogel | Intravitreal injection | Sustainable release patterns without any dose dumping | Not specified | Ranibizumab | [81] |
| Liposome-loaded hydrogel | Sub-tenon administration | High antiangiogenic properties, increased drug residency | Limited drug loading capacity and Short-term efficacy (up to 21 days) | Sunitinib, acriflavine | [82] |
| Peptide hydrogels | Intravitreal injection | Immediate improvement in reducing vascular leak | Limited follow-up time (14 days) | Pro-angiogenic and anti-angiogenic peptide | [83] |
| Peptide hydrogels | Intravitreal injection | Controlled drug release | Low bioavailability | Conbercept | [84] |
Future perspectives of gel-based drug delivery systems
Delivering therapeutic agents to the posterior eye is a formidable challenge due to the complex eye anatomy and limited understanding of underlying diseases. Effective drugs and delivery systems demand a deep understanding of cellular-level disease pathways. Ocular drug delivery technology has made substantial progress in treating posterior eye conditions. Traditional treatments involve injections or implants of agents like anti-VEGF and anti-inflammatory drugs. However, repeated invasive dosing raises concerns about compliance and financial burden for patients and clinicians alike.
In this context, less invasive ocular drug delivery systems are crucial, offering sustained drug release and enhanced effectiveness. Gel-based drug delivery systems reach drugs to the posterior eye tissues. This approach minimizes off-target effects, amplifying therapeutic impact and reducing side-effect risks. Sustained drug release from these systems eliminates the need for frequent dosing, greatly enhancing patient compliance and treatment outcomes. Moreover, gels serve as protective barriers, safeguarding delicate therapeutic agents from degradation and obviating the need for preservatives that might cause irritation.
Furthermore, gel-based systems offer the least invasive or non-invasive administration routes, such as topical application, minimizing patient discomfort and infection risks associated with invasive procedures [4]. Numerous clinical trials are evaluating various hydrogels’ safety, effectiveness, and stability for clinical use [85]. Additionally, implantable systems and nano-formulations show promise in ongoing studies. However, while preclinical research has progressed significantly, few ophthalmic drug delivery systems have transitioned to the clinical market.
Notably, Retisert and Ozurdex are FDA-approved intravitreal implants for treating uveitis, AMD, and macular edema. Retisert, containing fluocinolone acetonide, provides corticosteroid therapy for approximately 2.5 years in the affected eye’s posterior segment [86]. Ozurdex employs a biodegradable hydrogel to release dexamethasone gradually, reducing inflammation and suppressing immune responses [87].
Translating these delivery systems to clinical use faces challenges like enhancing therapeutic agent bioavailability, extending drug release duration, precise tissue targeting, adapting to varying eye conditions, improving drug stability, minimizing inflammation and irritation, designing user-friendly systems, curbing infection risks, exploring combination therapies, and focusing on personalized medicine. Regulatory approvals are vital to ensure widespread adoption of these innovative systems.
With continuous advancements in these domains, gel-based drug delivery systems can substantially enhance patient quality of life and revolutionize eye treatment standards. The future of ocular therapies for posterior eye diseases appears promising with the development of compound or hybrid nano-systems like nanoparticles and hydrogels, liposomes and dendrimers, stimuli-activated nano-systems, and nanocarriers combined with implants. The evolution of gel-based drug delivery systems presents exciting prospects in ophthalmology, offering long-term drug release, specific targeting, and improved patient adherence for various eye conditions. Ongoing research to refine these formulations will likely establish gel-based systems as key players in the future of eye care.
The data for this study were meticulously gathered through an exhaustive review of existing literature, research papers, and studies focused on hydrogel-based formulations for drug delivery to the posterior segment of the eye in the field of ophthalmology. Multiple reputable sources, including peer-reviewed journals, academic publications, conference proceedings, and respected online references, were consulted to compile pertinent data and gain comprehensive insights into the various applications and implications of hydrogel-based drug delivery in ocular health. In the beginning sections of this paper, we have evaluated the most routine and available methods to deliver drugs and biomolecules to the posterior segment of the eye. Also, we have assessed the post-segment action mechanisms, barriers, and limitations that are restricting drug delivery to this area. Clearly, we have concluded that hydrogel is an alternative to overcome barriers and effective drug delivery to deliver drug, gene, and macro/micro molecules to this impassable area. Afterward, we compared the pros and cons of hydrogel with other carriers and delivery methods. We have mentioned many successful studies and these studies conspicuously affirmed that hydrogels provided the delivery with higher drug availability, least invasive or non-invasive entrance, and better transmission through eye barriers. Emphasis was placed on selecting studies that presented diverse examples showcasing the applications of hydrogel-based drug delivery in addressing various aspects of ocular health. This rigorous methodology ensures the credibility and reliability of the data presented in this article.
- Bahmanpour AH, Ghaffari M, Ashraf S, Mozafari M. Nanoengineered biomaterials for diabetes. Nanoengineered Biomaterials for Advanced Drug Delivery. 2020; 735–752.
- Bahmanpour A, Ghaffari M, Milan PB, Moztarzadeh F, Mozafari M. Synthesis and characterization of thermosensitive hydrogel based on quaternized chitosan for intranasal delivery of insulin. Biotechnol Appl Biochem. 2021 Apr;68(2):247-256. doi: 10.1002/bab.1917. Epub 2020 May 20. PMID: 32250466.
- Sepahvandi A, Eskandari M, Moztarzadeh F. Drug delivery systems to the posterior segment of the eye: implants and nanoparticles. Bionanoscience. 2016; 6: 276–283.
- Sawarkar S, Pimple P, Sawant A, Nair S. Current insights into targeting strategies for the effective therapy of diseases of the posterior eye segment. Crit Rev Ther Drug Carrier Syst.
- Srirangam R, Hippalgaonkar K, Avula B, Khan IA, Majumdar S. Evaluation of the intravenous and topical routes for ocular delivery of hesperidin and hesperetin. J Ocul Pharmacol Ther. 2012 Dec;28(6):618-27. doi: 10.1089/jop.2012.0040. Epub 2012 Jul 13. PMID: 22794525; PMCID: PMC3505831.
- Kang-Mieler JJ, Osswald CR, Mieler WF. Advances in ocular drug delivery: emphasis on the posterior segment. Expert Opin Drug Deliv. 2014 Oct;11(10):1647-60. doi: 10.1517/17425247.2014.935338. Epub 2014 Jun 30. PMID: 24975820.
- Nayak K, Misra M. A review on recent drug delivery systems for posterior segment of eye. Biomed Pharmacother. 2018 Nov;107:1564-1582. doi: 10.1016/j.biopha.2018.08.138. Epub 2018 Sep 5. PMID: 30257375.
- Robinson MR, Lee SS, Kim H, Kim S, Lutz RJ, Galban C, Bungay PM, Yuan P, Wang NS, Kim J, Csaky KG. A rabbit model for assessing the ocular barriers to the transscleral delivery of triamcinolone acetonide. Exp Eye Res. 2006 Mar;82(3):479-87. doi: 10.1016/j.exer.2005.08.007. Epub 2005 Sep 15. PMID: 16168412.
- Ranta VP, Mannermaa E, Lummepuro K, Subrizi A, Laukkanen A, Antopolsky M, Murtomäki L, Hornof M, Urtti A. Barrier analysis of periocular drug delivery to the posterior segment. J Control Release. 2010 Nov 20;148(1):42-48. doi: 10.1016/j.jconrel.2010.08.028. Epub 2010 Sep 7. PMID: 20831888.
- Cholkar K, Dasari SR, Pal D, Mitra AK. Eye: Anatomy, physiology and barriers to drug delivery. in Ocular transporters and receptors. 2013; 1–36. Elsevier.
- Tabibian D, Hoogewoud F, Mavrakanas N, Schutz JS. Misdirected aqueous flow in rhegmatogenous retinal detachment: a pathophysiology update. Surv Ophthalmol. 2015 Jan-Feb;60(1):51-9. doi: 10.1016/j.survophthal.2014.07.002. Epub 2014 Aug 10. PMID: 25223495.
- del Amo EM, Vellonen KS, Kidron H, Urtti A. Intravitreal clearance and volume of distribution of compounds in rabbits: In silico prediction and pharmacokinetic simulations for drug development. Eur J Pharm Biopharm. 2015 Sep;95(Pt B):215-26. doi: 10.1016/j.ejpb.2015.01.003. Epub 2015 Jan 17. PMID: 25603198.
- Gabai A, Zeppieri M, Finocchio L, Salati C. Innovative Strategies for Drug Delivery to the Ocular Posterior Segment. Pharmaceutics. 2023 Jul 1;15(7):1862. doi: 10.3390/pharmaceutics15071862. PMID: 37514050; PMCID: PMC10385847.
- Qin Z, Yu X, Wu H, Yang L, Lv H, Yang X. Injectable and Cytocompatible Dual Cross-Linking Hydrogels with Enhanced Mechanical Strength and Stability. ACS Biomater Sci Eng. 2020 Jun 8;6(6):3529-3538. doi: 10.1021/acsbiomaterials.0c00416. Epub 2020 May 6. PMID: 33463187.
- Rahman MQ, Chuah KS, Macdonald EC, Trusler JP, Ramaesh K. The effect of pH, dilution, and temperature on the viscosity of ocular lubricants--shift in rheological parameters and potential clinical significance. Eye (Lond). 2012 Dec;26(12):1579-84. doi: 10.1038/eye.2012.211. Epub 2012 Oct 19. PMID: 23079749; PMCID: PMC3522845.
- Liu W, Lee BS, Mieler WF, Kang-Mieler JJ. Biodegradable Microsphere-Hydrogel Ocular Drug Delivery System for Controlled and Extended Release of Bioactive Aflibercept In Vitro. Curr Eye Res. 2019 Mar;44(3):264-274. doi: 10.1080/02713683.2018.1533983. Epub 2018 Oct 19. PMID: 30295090; PMCID: PMC7216294.
- Pan M, Ren Z, Ma X, Chen L, Lv G, Liu X, Li S, Li X, Wang J. A biomimetic peptide-drug supramolecular hydrogel as eyedrops enables controlled release of ophthalmic drugs. Acta Biomater. 2023 Sep 1;167:195-204. doi: 10.1016/j.actbio.2023.06.036. Epub 2023 Jun 29. PMID: 37392932.
- Santhanam S, Liang J, Struckhoff J, Hamilton PD, Ravi N. Biomimetic hydrogel with tunable mechanical properties for vitreous substitutes. Acta Biomater. 2016 Oct 1; 43:327-337. doi: 10.1016/j.actbio.2016.07.051. Epub 2016 Jul 29. PMID: 27481290; PMCID: PMC5787031.
- Lau CML, Chau Y. Injectable, hydrolytically degradable hydrogel for controllable, sustained protein release in the posterior eye. Invest Ophthalmol Vis Sci. 2019; 60: 5395.
- Mishra D, Gade S, Glover K, Sheshala R, Singh TRR. Vitreous Humor: Composition, Characteristics and Implication on Intravitreal Drug Delivery. Curr Eye Res. 2023 Feb;48(2):208-218. doi: 10.1080/02713683.2022.2119254. Epub 2022 Nov 28. PMID: 36036478.
- Abdelmohsen HAM, Copeland NA, Hardy JG. Light-responsive biomaterials for ocular drug delivery. Drug Deliv Transl Res. 2022; 1–24.
- Albadr AA, Tekko IA, Vora LK, Ali AA, Laverty G, Donnelly RF, Thakur RRS. Rapidly dissolving microneedle patch of amphotericin B for intracorneal fungal infections. Drug Deliv Transl Res. 2022 Apr;12(4):931-943. doi: 10.1007/s13346-021-01032-2. Epub 2021 Jul 23. PMID: 34302273; PMCID: PMC8888497.
- Wu Y, Vora LK, Wang Y, Adrianto MF, Tekko IA, Waite D, Donnelly RF, Thakur RRS. Long-acting nanoparticle-loaded bilayer microneedles for protein delivery to the posterior segment of the eye. Eur J Pharm Biopharm. 2021 Aug;165:306-318. doi: 10.1016/j.ejpb.2021.05.022. Epub 2021 May 26. PMID: 34048879.
- Lee K, Park S, Jo DH, Cho CS, Jang HY, Yi J, Kang M, Kim J, Jung HY, Kim JH, Ryu W, Khademhosseini A. Self-Plugging Microneedle (SPM) for Intravitreal Drug Delivery. Adv Healthc Mater. 2022 Jun;11(12):e2102599. doi: 10.1002/adhm.202102599. Epub 2022 Mar 3. PMID: 35192734.
- Roy G, Garg P, Venuganti VVK. Microneedle scleral patch for minimally invasive delivery of triamcinolone to the posterior segment of eye. Int J Pharm. 2022 Jan 25;612:121305. doi: 10.1016/j.ijpharm.2021.121305. Epub 2021 Nov 17. PMID: 34800618.
- Aguirre-Ramírez M, Silva-Jiménez H, Banat IM, Díaz De Rienzo MA. Surfactants: physicochemical interactions with biological macromolecules. Biotechnol Lett. 2021 Mar;43(3):523-535. doi: 10.1007/s10529-020-03054-1. Epub 2021 Feb 3. PMID: 33534014; PMCID: PMC7872986.
- Duan Y, Dhar A, Patel C, Khimani M, Neogi S, Sharma P, Siva Kumar N, Vekariya RL. A brief review on solid lipid nanoparticles: part and parcel of contemporary drug delivery systems. RSC Adv. 2020 Jul 17;10(45):26777-26791. doi: 10.1039/d0ra03491f. PMID: 35515778; PMCID: PMC9055574.
- Scioli Montoto S, Muraca G, Ruiz ME. Solid Lipid Nanoparticles for Drug Delivery: Pharmacological and Biopharmaceutical Aspects. Front Mol Biosci. 2020 Oct 30;7:587997. doi: 10.3389/fmolb.2020.587997. PMID: 33195435; PMCID: PMC7662460.
- Chauhan I, Yasir M, Verma M, Singh AP. Nanostructured Lipid Carriers: A Groundbreaking Approach for Transdermal Drug Delivery. Adv Pharm Bull. 2020 Jun;10(2):150-165. doi: 10.34172/apb.2020.021. Epub 2020 Feb 18. PMID: 32373485; PMCID: PMC7191226.
- Akulo KA, Adali T, Moyo MTG, Bodamyali T. Intravitreal Injectable Hydrogels for Sustained Drug Delivery in Glaucoma Treatment and Therapy. Polymers (Basel). 2022 Jun 10;14(12):2359. doi: 10.3390/polym14122359. PMID: 35745935; PMCID: PMC9230531.
- Arsenijevic Y, Berger A, Udry F, Kostic C. Lentiviral Vectors for Ocular Gene Therapy. Pharmaceutics. 2022 Jul 31;14(8):1605. doi: 10.3390/pharmaceutics14081605. PMID: 36015231; PMCID: PMC9414879.
- Deng C, Zhao PY, Branham K, Schlegel D, Fahim AT, Jayasundera TK, Khan N, Besirli CG. Real-world outcomes of voretigene neparvovec treatment in pediatric patients with RPE65-associated Leber congenital amaurosis. Graefes Arch Clin Exp Ophthalmol. 2022 May;260(5):1543-1550. doi: 10.1007/s00417-021-05508-2. Epub 2022 Jan 10. PMID: 35001204; PMCID: PMC9010358.
- Sisk R. Subretinal Delivery of RGX-314: A Gene Therapy for Neovascular Age-Related Macular Degeneration (nAMD). Invest Ophthalmol Vis Sci. 2023; 64: 5061.
- Parker MA, Erker LR, Audo I, Choi D, Mohand-Said S, Sestakauskas K, Benoit P, Appelqvist T, Krahmer M, Ségaut-Prévost C, Lujan BJ, Faridi A, Chegarnov EN, Steinkamp PN, Ku C, da Palma MM, Barale PO, Ayelo-Scheer S, Lauer A, Stout T, Wilson DJ, Weleber RG, Pennesi ME, Sahel JA, Yang P. Three-Year Safety Results of SAR422459 (EIAV-ABCA4) Gene Therapy in Patients With ABCA4-Associated Stargardt Disease: An Open-Label Dose-Escalation Phase I/IIa Clinical Trial, Cohorts 1-5. Am J Ophthalmol. 2022 Aug;240:285-301. doi: 10.1016/j.ajo.2022.02.013. Epub 2022 Mar 4. PMID: 35248547; PMCID: PMC9308722.
- Seah I, Ong C, Liu Z, Su X. Polymeric biomaterials in the treatment of posterior segment diseases. Front Med (Lausanne). 2022 Aug 18;9:949543. doi: 10.3389/fmed.2022.949543. PMID: 36059842; PMCID: PMC9433984.
- Reddy SK, Ballal AR, Shailaja S, Seetharam RN, Raghu CH, Sankhe R, Pai K, Tender T, Mathew M, Aroor A, Shetty AK, Adiga S, Devi V, Muttigi MS, Upadhya D. Small extracellular vesicle-loaded bevacizumab reduces the frequency of intravitreal injection required for diabetic retinopathy. Theranostics. 2023 Apr 9;13(7):2241-2255. doi: 10.7150/thno.78426. PMID: 37153730; PMCID: PMC10157735.
- Peng WY, He LW, Yin XF, Zhou BB, Zhou T, Zhou SY. Successful regression of newly formed corneal neovascularization by subconjunctival injection of bevacizumab in patients with chemical burns. Front Med (Lausanne). 2023 Jun 22;10:1210765. doi: 10.3389/fmed.2023.1210765. PMID: 37425330; PMCID: PMC10324651.
- Phasukkijwatana N, Jongpipatchai R, Phuksapaisalsilp P, Pharkjaksu S, Ngamskulrungroj P, Prakhunhungsit S. Effect of fenestrated sterile drape and face mask on bacterial dispersion toward the periocular area during intravitreal injection. Sci Rep. 2023 Jun 19;13(1):9878. doi: 10.1038/s41598-023-37091-3. PMID: 37336958; PMCID: PMC10279653.
- Li J, Mooney DJ. Designing hydrogels for controlled drug delivery. Nat Rev Mater. 2016 Dec;1(12):16071. doi: 10.1038/natrevmats.2016.71. Epub 2016 Oct 18. PMID: 29657852; PMCID: PMC5898614.
- Wang K, Han Z. Injectable hydrogels for ophthalmic applications. J Control Release. 2017 Dec 28;268:212-224. doi: 10.1016/j.jconrel.2017.10.031. Epub 2017 Oct 20. PMID: 29061512; PMCID: PMC5722685.
- Alonso JM, Andrade Del Olmo J, Perez Gonzalez R, Saez-Martinez V. Injectable Hydrogels: From Laboratory to Industrialization. Polymers (Basel). 2021 Feb 22;13(4):650. doi: 10.3390/polym13040650. PMID: 33671648; PMCID: PMC7926321.
- Doshi RR, Bakri SJ, Fung AE. Intravitreal injection technique. Semin Ophthalmol. 2011 May;26(3):104-13. doi: 10.3109/08820538.2010.541318. PMID: 21609222.
- Zhang Q, Weber C, Schubert US, Hoogenboom R. Thermoresponsive polymers with lower critical solution temperature: from fundamental aspects and measuring techniques to recommended turbidimetry conditions. Mater Horiz. 2017; 4 : 109–116.
- Gade S, Larraneta E, Donnelly RF, Vanrell RH, Alvarez‐Lorenzo C, Thakur RRS. Development of injectable thermoresponsive Cs‐g‐PNIPAAm hydrogel for intrascleral drug delivery of sunitinib malate for the posterior segment ocular disease, age‐related macular degeneration. Acta Ophthalmol. 2022; 100.
- Annala A, Ilochonwu BC, Wilbie D, Sadeghi A, Hennink WE, Vermonden T. Self-Healing Thermosensitive Hydrogel for Sustained Release of Dexamethasone for Ocular Therapy. ACS Polym Au. 2022 Nov 3;3(1):118-131. doi: 10.1021/acspolymersau.2c00038. PMID: 36785837; PMCID: PMC9912331.
- Meany EL. Injectable Polymer‐Nanoparticle Hydrogel for the Sustained Intravitreal Delivery of Bimatoprost. Adv Ther (Weinh). 2023; 6: 2200207.
- Bisht R, Jaiswal JK, Chen YS, Jin J, Rupenthal ID. Light-responsive in situ forming injectable implants for effective drug delivery to the posterior segment of the eye. Expert Opin Drug Deliv. 2016 Jul;13(7):953-62. doi: 10.1517/17425247.2016.1163334. Epub 2016 Mar 24. PMID: 26967153.
- Peng K, Tomatsu I, Kros A. Light controlled protein release from a supramolecular hydrogel. Chem Commun (Camb). 2010 Jun 21;46(23):4094-6. doi: 10.1039/c002565h. Epub 2010 May 13. PMID: 20464018.
- Bisht R, Jaiswal JK, Oliver VF, Eurtivong C, Reynisson J, Rupenthal ID. Preparation and evaluation of PLGA nanoparticle-loaded biodegradable light-responsive injectable implants as a promising platform for intravitreal drug delivery. J Drug Deliv Sci Technol. 2017; 40:142–156.
- Shen C, Zhao X, Ren Z, Yang B, Wang X, Hu A, Hu J. In Situ Formation of Injectable Gelatin Methacryloyl (GelMA) Hydrogels for Effective Intraocular Delivery of Triamcinolone Acetonide. Int J Mol Sci. 2023 Mar 4;24(5):4957. doi: 10.3390/ijms24054957. PMID: 36902389; PMCID: PMC10003315.
- Kolb HC, Finn MG, Sharpless KB. Click Chemistry: Diverse Chemical Function from a Few Good Reactions. Angew Chem Int Ed Engl. 2001 Jun 1;40(11):2004-2021. doi: 10.1002/1521-3773(20010601)40:11<2004::AID-ANIE2004>3.0.CO;2-5. PMID: 11433435.
- Yu Y, Lau LC, Lo AC, Chau Y. Injectable Chemically Crosslinked Hydrogel for the Controlled Release of Bevacizumab in Vitreous: A 6-Month In Vivo Study. Transl Vis Sci Technol. 2015 Mar 10;4(2):5. doi: 10.1167/tvst.4.2.5. PMID: 25774331; PMCID: PMC4356035.
- Ilochonwu BC, Mihajlovic M, Maas-Bakker RF, Rousou C, Tang M, Chen M, Hennink WE, Vermonden T. Hyaluronic Acid-PEG-Based Diels-Alder In Situ Forming Hydrogels for Sustained Intraocular Delivery of Bevacizumab. Biomacromolecules. 2022 Jul 11;23(7):2914-2929. doi: 10.1021/acs.biomac.2c00383. Epub 2022 Jun 23. PMID: 35735135; PMCID: PMC9277588.
- Zhuo S, Zhang F, Yu J, Zhang X, Yang G, Liu X. pH-Sensitive Biomaterials for Drug Delivery. Molecules. 2020 Nov 30;25(23):5649. doi: 10.3390/molecules25235649. PMID: 33266162; PMCID: PMC7730929.
- Yu S, Zhang X, Tan G, Tian L, Liu D, Liu Y, Yang X, Pan W. A novel pH-induced thermosensitive hydrogel composed of carboxymethyl chitosan and poloxamer cross-linked by glutaraldehyde for ophthalmic drug delivery. Carbohydr Polym. 2017 Jan 2;155:208-217. doi: 10.1016/j.carbpol.2016.08.073. Epub 2016 Aug 25. PMID: 27702506.
- Jalababu R, Reddy MK, Reddy KVNS, Rao KSVK. Hydrogels as Smart Drug Delivery Systems: Recent Advances. Smart Nanomaterials in Biomedical Applications.2022; 173–201.
- Xu H, Liu Y, Jin L, Chen X, Chen X, Wang Q, Tang Z. Preparation and Characterization of Ion-Sensitive Brimonidine Tartrate In Situ Gel for Ocular Delivery. Pharmaceuticals (Basel). 2023 Jan 8;16(1):90. doi: 10.3390/ph16010090. PMID: 36678587; PMCID: PMC9866900.
- Karmakar S, Manna S, Kabiraj S, Jana S. Recent progress in alginate-based carriers for ocular targeting of therapeutics. Food Hydrocolloids for Health. 2022; 2:100071.
- Rupenthal ID, Green CR, Alany RG. Comparison of ion-activated in situ gelling systems for ocular drug delivery. Part 1: physicochemical characterisation and in vitro release. Int J Pharm. 2011 Jun 15;411(1-2):69-77. doi: 10.1016/j.ijpharm.2011.03.042. Epub 2011 Mar 29. PMID: 21453762.
- Dromel PC, Singh D, Alexander-Katz A, Kurisawa M, Spector M, Young M. Mechano-Chemical Effect of Gelatin- and HA-Based Hydrogels on Human Retinal Progenitor Cells. Gels. 2023 Jan 11;9(1):58. doi: 10.3390/gels9010058. PMID: 36661824; PMCID: PMC9858647.
- Yu S, Wang S, Xia L, Hu H, Zou M, Jiang Z, Chi J, Zhang Y, Li H, Yang C, Liu W, Han B. Injectable self-crosslinking hydrogels based on hyaluronic acid as vitreous substitutes. Int J Biol Macromol. 2022 May 31;208:159-171. doi: 10.1016/j.ijbiomac.2022.03.046. Epub 2022 Mar 14. PMID: 35301003.
- Song Y, Nagai N, Saijo S, Kaji H, Nishizawa M, Abe T. In situ formation of injectable chitosan-gelatin hydrogels through double crosslinking for sustained intraocular drug delivery. Mater Sci Eng C Mater Biol Appl. 2018 Jul 1;88:1-12. doi: 10.1016/j.msec.2018.02.022. Epub 2018 Mar 6. PMID: 29636124.
- Bellotti E, Fedorchak MV, Velankar S, Little SR. Tuning of thermoresponsive pNIPAAm hydrogels for the topical retention of controlled release ocular therapeutics. J Mater Chem B. 2019 Feb 28;7(8):1276-1283. doi: 10.1039/C8TB02976H. Epub 2019 Jan 25. PMID: 30931126; PMCID: PMC6437675.
- Zhu M, Wang J, Li N. A novel thermo-sensitive hydrogel-based on poly(N-isopropylacrylamide)/hyaluronic acid of ketoconazole for ophthalmic delivery. Artif Cells Nanomed Biotechnol. 2018 Sep;46(6):1282-1287. doi: 10.1080/21691401.2017.1368024. Epub 2017 Aug 21. PMID: 28826241.
- Seo JS, Tumursukh NE, Choi JH, Song Y, Jeon G, Kim NE, Kim SJ, Kim N, Song JE, Khang G. Modified gellan gum-based hydrogel with enhanced mechanical properties for application as a cell carrier for cornea endothelial cells. Int J Biol Macromol. 2023 May 1;236:123878. doi: 10.1016/j.ijbiomac.2023.123878. Epub 2023 Mar 7. PMID: 36894057.
- Wong FSY, Tsang KK, Chu AMW, Chan BP, Yao KM, Lo ACY. Injectable cell-encapsulating composite alginate-collagen platform with inducible termination switch for safer ocular drug delivery. Biomaterials. 2019 May;201:53-67. doi: 10.1016/j.biomaterials.2019.01.032. Epub 2019 Feb 5. PMID: 30797114.
- Terreni E, Zucchetti E, Tampucci S, Burgalassi S, Monti D, Chetoni P. Combination of Nanomicellar Technology and In Situ Gelling Polymer as Ocular Drug Delivery System (ODDS) for Cyclosporine-A. Pharmaceutics. 2021 Feb 1;13(2):192. doi: 10.3390/pharmaceutics13020192. PMID: 33535607; PMCID: PMC7912864.
- Chen Q. An injectable thermosensitive hydrogel encapsulating tetramethylpyrazine nanocrystals alleviates angiogenesis and apoptosis in a choroidal neovascularization mouse model. Appl Mater Today.2023; 33:101867.
- Su W, Liu C, Jiang X, Lv Y, Chen Q, Shi J, Zhang H, Ma Q, Ge C, Kong F, Li X, Liu Y, Chen Y, Qu D. An intravitreal-injectable hydrogel depot doped borneol-decorated dual-drug-coloaded microemulsions for long-lasting retina delivery and synergistic therapy of wAMD. J Nanobiotechnology. 2023 Mar 1;21(1):71. doi: 10.1186/s12951-023-01829-y. PMID: 36859261; PMCID: PMC9976542.
- Awwad S, Abubakre A, Angkawinitwong U, Khaw PT, Brocchini S. In situ antibody-loaded hydrogel for intravitreal delivery. Eur J Pharm Sci. 2019 Sep 1;137:104993. doi: 10.1016/j.ejps.2019.104993. Epub 2019 Jul 11. PMID: 31302214.
- Abedin Zadeh M, Alany RG, Satarian L, Shavandi A, Abdullah Almousa M, Brocchini S, Khoder M. Maillard Reaction Crosslinked Alginate-Albumin Scaffolds for Enhanced Fenofibrate Delivery to the Retina: A Promising Strategy to Treat RPE-Related Dysfunction. Pharmaceutics. 2023 Apr 24;15(5):1330. doi: 10.3390/pharmaceutics15051330. PMID: 37242572; PMCID: PMC10224349.
- Thirupathi K, Phan TTV, Santhamoorthy M, Ramkumar V, Kim SC. pH and Thermoresponsive PNIPAm-co-Polyacrylamide Hydrogel for Dual Stimuli-Responsive Controlled Drug Delivery. Polymers (Basel). 2022 Dec 29;15(1):167. doi: 10.3390/polym15010167. PMID: 36616517; PMCID: PMC9823768.
- Khan MS, Ravi PR, Mir SI, Rawat PS. Optimization and in vivo evaluation of triamcinolone acetonide loaded in situ gel prepared using reacted tamarind seed xyloglucan and kappa-carrageenan for ocular delivery. Int J Biol Macromol. 2023 Apr 1;233:123533. doi: 10.1016/j.ijbiomac.2023.123533. Epub 2023 Feb 3. PMID: 36740111.
- Liu W, Tawakol AP, Rudeen KM, Mieler WF, Kang-Mieler JJ. Treatment Efficacy and Biocompatibility of a Biodegradable Aflibercept-Loaded Microsphere-Hydrogel Drug Delivery System. Transl Vis Sci Technol. 2020 Oct 13;9(11):13. doi: 10.1167/tvst.9.11.13. PMID: 33117605; PMCID: PMC7571288.
- Gade SS, Pentlavalli S, Mishra D, Vora LK, Waite D, Alvarez-Lorenzo CI, Herrero Vanrell MR, Laverty G, Larraneta E, Donnelly RF, Thakur RRS. Injectable Depot Forming Thermoresponsive Hydrogel for Sustained Intrascleral Delivery of Sunitinib Using Hollow Microneedles. J Ocul Pharmacol Ther. 2022 Jul-Aug;38(6):433-448. doi: 10.1089/jop.2022.0016. PMID: 35914241.
- Gao H, Chen M, Liu Y, Zhang D, Shen J, Ni N, Tang Z, Ju Y, Dai X, Zhuang A, Wang Z, Chen Q, Fan X, Liu Z, Gu P. Injectable Anti-Inflammatory Supramolecular Nanofiber Hydrogel to Promote Anti-VEGF Therapy in Age-Related Macular Degeneration Treatment. Adv Mater. 2023 Jan;35(2):e2204994. doi: 10.1002/adma.202204994. Epub 2022 Dec 11. PMID: 36349821.
- Goswami M, Sadasivam R, Packirisamy G. Viability studies of hydrogel contact lens on a 3D printed platform as ocular drug delivery carrier for diabetic retinopathy. Mater Lett. 2023; 333:133636.
- Sadasivam R, Packirisamy G, Goswami M. Biocompatible soft hydrogel lens as topical implants for diabetic retinopathy. Mater Lett. 2022; 318:132174.
- Ottonelli I, Bighinati A, Adani E, Loll F, Caraffi R, Vandelli MA, Boury F, Tosi G, Duskey JT, Marigo V, Ruozi B. Optimization of an Injectable Hydrogel Depot System for the Controlled Release of Retinal-Targeted Hybrid Nanoparticles. Pharmaceutics. 2022 Dec 21;15(1):25. doi: 10.3390/pharmaceutics15010025. PMID: 36678654; PMCID: PMC9862926.
- Taheri SL, Rezazadeh M, Hassanzadeh F, Akbari V, Dehghani A, Talebi A, Mostafavi SA. Preparation, physicochemical, and retinal anti-angiogenic evaluation of poloxamer hydrogel containing dexamethasone/avastin-loaded chitosan-N-acetyl-L-cysteine nanoparticles. Int J Biol Macromol. 2022 Nov 1;220:1605-1618. doi: 10.1016/j.ijbiomac.2022.09.101. Epub 2022 Sep 16. PMID: 36116595.
- Yaylaci S, Dinç E, Aydın B, Tekinay AB, Guler MO. Peptide Nanofiber System for Sustained Delivery of Anti-VEGF Proteins to the Eye Vitreous. Pharmaceutics. 2023 Apr 18;15(4):1264. doi: 10.3390/pharmaceutics15041264. PMID: 37111749; PMCID: PMC10141348.
- Li J. A novel, liposome-loaded, injectable hydrogel for enhanced treatment of choroidal neovascularization by sub-tenon’s injection. Mater Today Nano.2022; 20:100264.
- Acevedo-Jake A, Shi S, Siddiqui Z, Sanyal S, Schur R, Kaja S, Yuan A, Kumar VA. Preclinical Efficacy of Pro- and Anti-Angiogenic Peptide Hydrogels to Treat Age-Related Macular Degeneration. Bioengineering (Basel). 2021 Nov 23;8(12):190. doi: 10.3390/bioengineering8120190. PMID: 34940343; PMCID: PMC8698576.
- Fan W, Li S, Tao J, Yu C, Sun M, Xie Z, Wu X, Ge L, Wu Y, Liu Y. Anti-Vascular Endothelial Growth Factor Drug Conbercept-Loaded Peptide Hydrogel Reduced Angiogenesis in the Neovascular Age-Related Macular Degeneration. J Biomed Nanotechnol. 2022 Jan 1;18(1):277-287. doi: 10.1166/jbn.2022.3227. PMID: 35180922.
- Hashida N, Nishida K. Recent advances and future prospects: Current status and challenges of the intraocular injection of drugs for vitreoretinal diseases. Adv Drug Deliv Rev. 2023 Jul;198:114870. doi: 10.1016/j.addr.2023.114870. Epub 2023 May 10. PMID: 37172783.
- Jaffe GJ, Martin D, Callanan D, Pearson PA, Levy B, Comstock T; Fluocinolone Acetonide Uveitis Study Group. Fluocinolone acetonide implant (Retisert) for noninfectious posterior uveitis: thirty-four-week results of a multicenter randomized clinical study. Ophthalmology. 2006 Jun;113(6):1020-7. doi: 10.1016/j.ophtha.2006.02.021. Epub 2006 May 9. PMID: 16690128.
- Costello MA, Liu J, Wang Y, Qin B, Xu X, Li Q, Lynd NA, Zhang F. Reverse engineering the Ozurdex dexamethasone intravitreal implant. Int J Pharm. 2023 Mar 5;634:122625. doi: 10.1016/j.ijpharm.2023.122625. Epub 2023 Jan 20. PMID: 36690129.