Figure 0
Mesoscopic irreversible thermodynamics of morphological evolution kinetics of helical conformation in bioproteins ‘DNA’ under the isothermal isobaric conditions
Tarik Omer Ogurtani* and Ersin Emre Oren
Published: 11 March, 2020 | Volume 4 - Issue 1 | Pages: 009-019
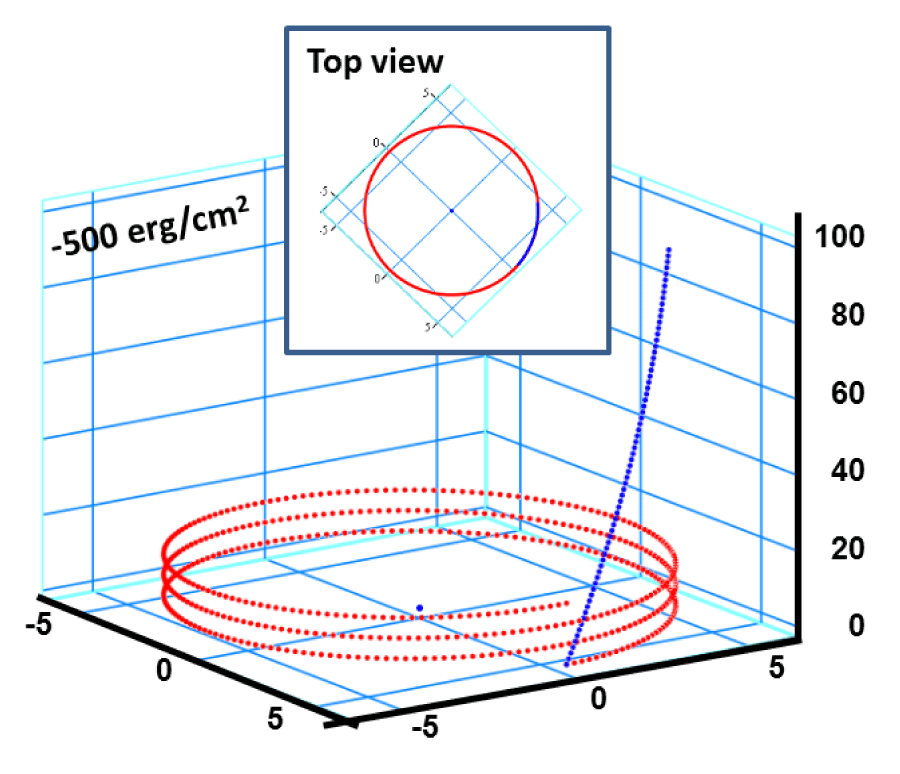
Figure 0:
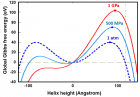
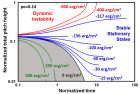
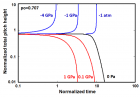
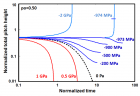
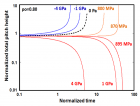
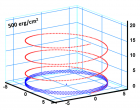
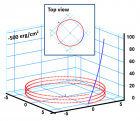
a) Perspective view: Helical conformation of α - peptide at the stationary state is illustrated by (red dotted line), which has pitch height 5.4Ao, and radius 6Ao and 3 rings. When it is exposed to the spontaneous relaxation under the negative interfacial Gibbs free energy of gs = -500 erg/cm2 [p = -1.677GPa uniaxial tension, isobaric] the unfolding takes place (blue line), which is found to be 99.9% of the full transition b) Top View: Partial unfolding appears as an arc segment (blue color) having Θ = 49.5o azimuthal angle with a torsional inclination angle of β = 2.563o. That should be zero if one takes final total pitch height as p = lo.
Read Full Article HTML DOI: 10.29328/journal.abse.1001008 Cite this Article Read Full Article PDF
More Images
Similar Articles
-
Peptide-based antifouling aptasensor for cardiac troponin I detection by surface plasmon resonance applied in medium sized Myocardial InfarctionJen-Tsai Liu*,Yi Wu,Jia Xin Che,Shwu Jen Chang,Ching-Jung Chen*. Peptide-based antifouling aptasensor for cardiac troponin I detection by surface plasmon resonance applied in medium sized Myocardial Infarction. . 2020 doi: 10.29328/journal.abse.1001007; 4: 001-008
Recently Viewed
-
Impact of Latex Sensitization on Asthma and Rhinitis Progression: A Study at Abidjan-Cocody University Hospital - Côte d’Ivoire (Progression of Asthma and Rhinitis related to Latex Sensitization)Dasse Sery Romuald*, KL Siransy, N Koffi, RO Yeboah, EK Nguessan, HA Adou, VP Goran-Kouacou, AU Assi, JY Seri, S Moussa, D Oura, CL Memel, H Koya, E Atoukoula. Impact of Latex Sensitization on Asthma and Rhinitis Progression: A Study at Abidjan-Cocody University Hospital - Côte d’Ivoire (Progression of Asthma and Rhinitis related to Latex Sensitization). Arch Asthma Allergy Immunol. 2024: doi: 10.29328/journal.aaai.1001035; 8: 007-012
-
Eyes and Minds under Siege: How Digital Exposure Is Threatening Ocular and Neural Health in AdolescentsRabia Nizam*,Alina Rizwan,Arwa Anjum,Hejab Khan. Eyes and Minds under Siege: How Digital Exposure Is Threatening Ocular and Neural Health in Adolescents. J Community Med Health Solut. 2025: doi: 10.29328/journal.jcmhs.1001059; 6: 053-054
-
From the Single Bacterial Cell to the Microbial Community: A Round Trip to better understand the Secrets of Complex Microbiological EcosystemsErasmo Neviani*. From the Single Bacterial Cell to the Microbial Community: A Round Trip to better understand the Secrets of Complex Microbiological Ecosystems. Int J Clin Microbiol Biochem Technol. 2024: doi: 10.29328/journal.ijcmbt.1001029; 7: 006-008
-
Cancer Cell Resistance: The Emergent Intelligence of Adaptation and the Need for Biophysical IntegrationMohamed H Doweidar*. Cancer Cell Resistance: The Emergent Intelligence of Adaptation and the Need for Biophysical Integration. Int J Clin Microbiol Biochem Technol. 2025: doi: 10.29328/journal.ijcmbt.1001031; 8: 007-008
-
Analysis and Control of a Suicide Dynamics ModelLakshmi N Sridhar*. Analysis and Control of a Suicide Dynamics Model. Arch Psychiatr Ment Health. 2025: doi: 10.29328/journal.apmh.1001059; 9: 022-028
Most Viewed
-
Feasibility study of magnetic sensing for detecting single-neuron action potentialsDenis Tonini,Kai Wu,Renata Saha,Jian-Ping Wang*. Feasibility study of magnetic sensing for detecting single-neuron action potentials. Ann Biomed Sci Eng. 2022 doi: 10.29328/journal.abse.1001018; 6: 019-029
-
Evaluation of In vitro and Ex vivo Models for Studying the Effectiveness of Vaginal Drug Systems in Controlling Microbe Infections: A Systematic ReviewMohammad Hossein Karami*, Majid Abdouss*, Mandana Karami. Evaluation of In vitro and Ex vivo Models for Studying the Effectiveness of Vaginal Drug Systems in Controlling Microbe Infections: A Systematic Review. Clin J Obstet Gynecol. 2023 doi: 10.29328/journal.cjog.1001151; 6: 201-215
-
Causal Link between Human Blood Metabolites and Asthma: An Investigation Using Mendelian RandomizationYong-Qing Zhu, Xiao-Yan Meng, Jing-Hua Yang*. Causal Link between Human Blood Metabolites and Asthma: An Investigation Using Mendelian Randomization. Arch Asthma Allergy Immunol. 2023 doi: 10.29328/journal.aaai.1001032; 7: 012-022
-
An algorithm to safely manage oral food challenge in an office-based setting for children with multiple food allergiesNathalie Cottel,Aïcha Dieme,Véronique Orcel,Yannick Chantran,Mélisande Bourgoin-Heck,Jocelyne Just. An algorithm to safely manage oral food challenge in an office-based setting for children with multiple food allergies. Arch Asthma Allergy Immunol. 2021 doi: 10.29328/journal.aaai.1001027; 5: 030-037
-
Impact of Latex Sensitization on Asthma and Rhinitis Progression: A Study at Abidjan-Cocody University Hospital - Côte d’Ivoire (Progression of Asthma and Rhinitis related to Latex Sensitization)Dasse Sery Romuald*, KL Siransy, N Koffi, RO Yeboah, EK Nguessan, HA Adou, VP Goran-Kouacou, AU Assi, JY Seri, S Moussa, D Oura, CL Memel, H Koya, E Atoukoula. Impact of Latex Sensitization on Asthma and Rhinitis Progression: A Study at Abidjan-Cocody University Hospital - Côte d’Ivoire (Progression of Asthma and Rhinitis related to Latex Sensitization). Arch Asthma Allergy Immunol. 2024 doi: 10.29328/journal.aaai.1001035; 8: 007-012

If you are already a member of our network and need to keep track of any developments regarding a question you have already submitted, click "take me to my Query."